ω端带有四氢化类异戊二烯侧链的天然产物的分离和结构测定。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
氢化类异戊二烯存在于一系列具有重要生物学意义的天然产物中,如类异戊二烯醌、叶绿素、维生素E和醇。本研究建立了一种新的方法来测定两种天然产物(七戊烯环- 14e, 18e -二烯和(22R,5E,9E,13E,17E)-6,10,14,18,22,26-六甲基七元-5,9,13,17-四烯-2- 1)的四氢化类异戊二烯(THI)结构的手性,这些结构的手性碳中心从未被证实过。课课组先前从绿酚分枝杆菌中分离到前一种倍四烯,而本研究从woesei Conexibacter woesei中分离到后一种新的聚戊烯基丙酮。为了确定它们的手性,我们对这两种含硫萜类化合物进行臭氧分解,得到(R)-6,10-二甲基-2-十一烷酮。通过还原(S,E)-6,10-二甲基-2-十一烷,制备了具有光学活性的(S)-6,10-二甲基-2-十一烷作为真正的手性样品。对臭氧分解产物和正品(S)-和外消旋6,10-二甲基-2-十一烷酮样品的手性高效液相色谱分析明确了这两种化合物的THI手性碳中心为r,这种新方法可用于测定其他This的手性。这也有望有助于我们对THIs的生物合成机制和生物学作用的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
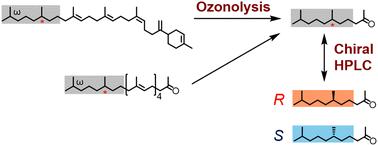
Isolation and structural determination of natural products bearing tetrahydrogenated isoprenoid side-chains at their ω-termini†
Hydrogenated isoprenoids are found in a range of biologically important natural products, such as isoprenoid quinones, chlorophyll, vitamin E, and dolichol. In this study, a new method was developed for determining the chirality of the tetrahydrogenated isoprenoid (THI) structures of two natural products, namely heptaprenylcycli-14E,18E-diene and (22R,5E,9E,13E,17E)-6,10,14,18,22,26-hexamethylheptacosa-5,9,13,17-tetraen-2-one, for which the chiral carbon centres have never been elucidated. Our research group previously isolated the former sesquarterpene from Mycobacterium chlorophenolicum, while the latter was isolated as a new polyprenyl acetone from Conexibacter woesei in the current study. To determine their chiralities, these two THI-containing terpenoids were subjected to ozonolysis to produce (R)-6,10-dimethyl-2-undecanones. The optically active (S)-6,10-dimethyl-2-undecanone was prepared as an authentic chiral sample by the reduction of (S,E)-6,10-dimethylundeca-3,9-dien-2-one. Chiral high-performance liquid chromatographic analysis of the ozonolysis products and of authentic (S)- and racemic 6,10-dimethyl-2-undecanone samples unambiguously assigned the THI chiral carbon centres of both compounds as R. This new method could be a useful tool for determining the chiralities of other THIs. It is also expected to contribute to our understanding of the biosynthetic mechanisms and biological roles of THIs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


