可再生天然气氧化蒸汽重整制氢用于小规模氨合成的镍硅催化剂
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
合成氨是一个能源密集型过程,每年消耗全球约1.8%的能源产出,产生约5亿吨二氧化碳。氢气的生产占氨合成过程所需总能量的80%以上。Haber-Bosch是氨生产中应用最广泛的技术;然而,它只有在经济上大规模可行。氧化蒸汽重整(OSR)是一种小型氨生产合成气的替代技术。OSR结合了甲烷的催化部分氧化(CPOM)和甲烷的蒸汽重整(SMR),有效地生成了H2/N2摩尔比为3的重整产物,这是合成氨所需的化学计量值。OSR面临的主要挑战之一是开发用于CPOM的催化材料,该材料不仅要具有高活性和稳定性,而且要具有因焦炭形成而失活的显著弹性。在本研究中,我们利用溶胶-凝胶法合成了一种新型廉价的镍基催化材料,并在低于500°C的温度下对CPOM进行了测试。所制备的催化剂具有与贵金属催化剂相当的显著活性,并且具有优异的抗积碳能力。催化活性被认为是由于羧基和缺氧区的存在,分别导致CH4和O2的吸附/活化增强。通过在450°C的CPOM阶段使用该催化材料,在800°C的SMR阶段使用氧化铝负载的镍催化剂,我们证明了OSR反应器生产具有合适H2/N2摩尔比的重整产物用于氨生产的可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
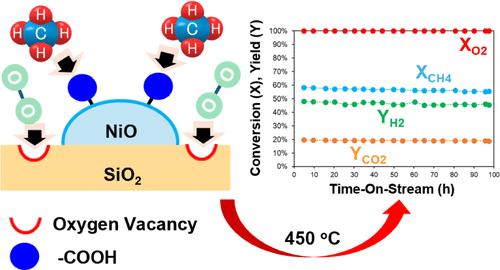
Nickel–Silicon Catalysts for the Oxidative Steam Reforming of Renewable Natural Gas to Produce Hydrogen for Small-Scale Ammonia Synthesis
The synthesis of ammonia is an energy-intensive process that consumes around 1.8% of global energy output each year and produces approximately 500 MMT of carbon dioxide. The production of hydrogen accounts for over 80% of the total energy required by the ammonia synthesis process. Haber-Bosch is the most widely used technology for ammonia production; however, it is only economically viable at a large scale. Oxidative steam reforming (OSR) is an alternative technology to produce synthesis gas for small-scale ammonia production. OSR combines the catalytic partial oxidation of methane (CPOM) and the steam reforming of methane (SMR) to efficiently generate a reformate with an H2/N2 molar ratio of 3, which is the stoichiometric value required for ammonia synthesis. One of the major challenges with OSR is the development of a catalytic material for CPOM that can show not only high activity and stability but also significant resilience to deactivation due to coke formation. In the present work, a novel and inexpensive nickel-based catalytic material has been synthesized using a facile sol–gel approach and tested for CPOM at temperatures below 500 °C. The prepared catalyst shows a remarkable activity comparable to that of catalysts based on noble metals and an outstanding resistance to the formation of carbon deposits. The catalytic activity is believed to result from the presence of carboxyl groups and oxygen-depleted regions, which led to an enhancement in the adsorption/activation of CH4 and O2, respectively. By using this catalytic material in the CPOM stage at 450 °C and an alumina-supported nickel catalyst in the SMR stage at 800 °C, we demonstrated the viability of an OSR reactor to produce a reformate with a suitable H2/N2 molar ratio for ammonia production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


