水对异戊二烯与铁取代隐黑烷非均相反应的抑制作用
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
生物源异戊二烯的运输和转化对对流层有机碳循环至关重要。铁取代隐黑烷是一种典型的高氧化电位矿物,它将异戊二烯表面单层氧化成甲酸和乙酸,同时结构中的Mn4+离子被还原成Mn3+和Mn2+。即使在较低的相对湿度(10%)下,水在异戊二烯中的流动也显著降低了异戊二烯的吸附和氧化。由于物理吸附的H2O保留了fe -取代隐黑烷的晶体结构和氧化能力,当异戊二烯流动中没有H2O时,吸附和氧化能力恢复。在(001)表面上的理论计算表明,异戊二烯更倾向于被Fe3+吸附,H2O倾向于形成氢键。由于水和异戊二烯的总吸附能降低,铁取代隐黑烷有利于湿润异戊二烯流动中水的吸附。异戊二烯在环境相对湿度下的低氧化性能表明,矿物粉尘在夜间被气溶胶直接氧化可能不是生物源异戊二烯的转化途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
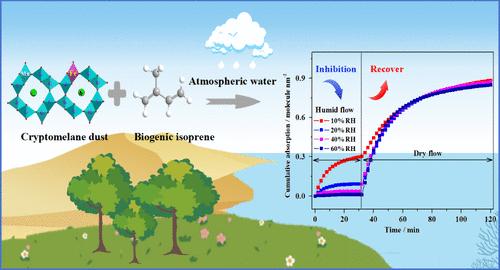
Inhibition Effect of H2O on the Heterogeneous Reaction between Isoprene and Fe-Substituted Cryptomelane
The transportation and transformation of biogenic isoprene are vital for the organic carbon cycle in the troposphere. As a typical mineral with high oxidation potential, Fe-substituted cryptomelane oxidizes the surface monolayer of isoprene into formic and acetic acids, and simultaneously, the Mn4+ ions in the structure are reduced to Mn3+ and Mn2+. The flow of H2O in isoprene decreases the adsorption and oxidation of isoprene significantly, even at low relative humidity (10%). As physisorbed H2O retains Fe-substituted cryptomelane’s crystal structure and oxidation ability, the adsorption and oxidation capacity recovers when H2O is absent in the isoprene flow. Theoretical calculations on (001) surfaces show that isoprene prefers to be adsorbed by the Fe3+ site and H2O tends to form hydrogen bonds. Due to the decrease in total adsorption energy of H2O and isoprene, Fe-substituted cryptomelane favors the adsorption of H2O in the flow of humid isoprene. The low oxidation performance at ambient relative humidity suggests that direct oxidation by aerosols of mineral dust might not be the transformation pathway of biogenic isoprene at night.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


