混合溶剂体系中质子活性和酸解离平衡的预测及其对乙酰丙酸酯化动力学的影响
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究提出了一种预测酯化反应动力学的新的热力学建模方法。详细研究了以硫酸为均相催化剂,在323 ~ 353 K的常压条件下,不同醇对乙酰丙酸的酯化反应。温度、溶剂和助溶剂对LA酯化反应的动力学和平衡有显著的影响。在这项工作中开发的新型建模方法通过关注催化剂对反应动力学的热力学描述,将绝对质子活度引入反应形式。这需要精确建模质子活度、吉布斯溶剂化能和酸解离平衡的混合水-有机反应体系,随着反应的进行改变组成。本文应用电解质状态方程ePC-SAFT来预测混合溶剂体系中质子的绝对活度。根据质子溶剂化吉布斯能和酸解离平衡的文献数据调整了ePC-SAFT参数。新方法在计算混合溶剂体系中质子的溶剂化吉布斯能、酸解离平衡和绝对质子活度方面具有很高的准确性。它可以定量地解释水对动力学的不利影响,尽管在水溶剂中酸解离很强,而质子活性很低。最终,这可以预测催化剂、溶剂和助溶剂加成对酯化动力学和平衡的影响,表明助溶剂γ-戊内酯对动力学的影响很小,而水显著降低反应速率,硫酸加成加速反应,这取决于硫酸的浓度。总之,这种方法通过可靠地预测关键反应条件对化学反应动力学和平衡的影响,可以减少实验工作量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
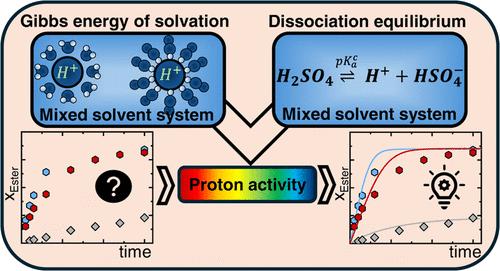
Predicting Proton Activity and Acid Dissociation Equilibria in Mixed-Solvent Systems, and Their Impact on Esterification Kinetics of Levulinic Acid
This study presents a novel thermodynamic modeling approach for predicting the reaction kinetics of esterification reactions. The system studied in detail was levulinic acid esterification by various alcohols, using sulfuric acid as a homogeneous catalyst at reaction temperatures between 323 and 353 K at atmospheric pressure. The effects of temperature, solvent, and cosolvent on the kinetics and equilibrium of LA esterification were found to be significant. The novel modeling approach developed in this work introduces absolute proton activity into reaction formalism by focusing on a thermodynamic description of the catalyst effects on reaction kinetics. That required accurate modeling of proton activities, Gibbs energies of solvation, and acid dissociation equilibria in the mixed aqueous–organic reaction system that changes composition with reaction progress. In this work, the electrolyte equation of state ePC-SAFT was applied to predict absolute proton activities in mixed solvent systems. The ePC-SAFT parameters were adjusted by literature-based data on proton Gibbs energies of solvation and on acid dissociation equilibria. The new approach allowed very good accuracy in calculating proton Gibbs energies of solvation, acid dissociation equilibria, and absolute proton activity in mixed-solvent systems. It could quantitatively explain the disadvantageous effect of water on kinetics, despite acid dissociation in water solvent is strong while proton activity is very low. Ultimately, this allowed predicting the influence of catalyst, solvent and of cosolvent addition on the kinetics and the equilibrium of the esterification, demonstrating that the cosolvent γ-valerolactone had minimal effect on the kinetics while water significantly decreased the reaction rate and sulfuric acid addition accelerated the reaction depending on sulfuric acid concentration. To conclude, this approach allows for a reduction in experimental effort by reliably predicting the influence of the key reaction conditions on kinetics and equilibria of chemical reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


