手性二茂铁P, P配体的高效固定化策略及不对称催化性能的增强
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
手性催化剂的固定化使其具有成本效益的重复使用,并简化了产品分离。然而,经典的共价键固定策略通常需要额外的步骤来修饰配体或载体。本文通过一步Friedel-Crafts反应将两种典型的手性二茂铁配体和三种相应的金属配合物固定在市售苯乙烯基聚合物上。我们的方法能够在不需要预修饰配体或载体的情况下方便地固定,并且代表了一种快速获得含芳基的异相配体或配体/金属配合物的直接策略。得到的非均相催化剂Taniaphos/Cu(Ι)和Zhaophos/Rh(Ι)在不对称烷基化反应和加氢反应中表现出优异的活性,产率分别高达99 %和98 %的对映体过量(e.e)。值得注意的是,Zhaophos/Ir(Ι)非均相催化剂在苯并恶嗪酮的不对称加氢反应中表现出两倍以上的性能。而且,所有的产物都可以不经色谱纯化而得到,核磁共振纯度在95% %以上。循环试验和连续流动实验表明,该多相催化剂可重复使用至少5次而不降低活性,或连续运行100 h而不降低活性,周转次数超过1875次,反应时间从24 h减少到4.7 min。本文章由计算机程序翻译,如有差异,请以英文原文为准。

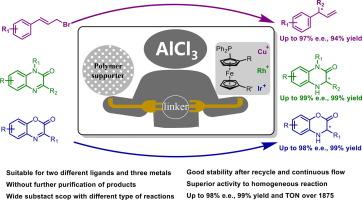
A highly efficient immobilization strategy for chiral ferrocene P, P-ligands with enhanced performance in asymmetric catalysis
The immobilization of chiral catalyst enables a cost-effective reuse and simplifies product separation. However, the classical covalent bonding immobilization strategies often require additional steps to modify the ligands or supports. Herein, an immobilization of two typical chiral ferrocene ligands and three corresponding metal complexes onto commercially available styrene-based polymers has been achieved via a one-step Friedel-Crafts reaction. Our method enables facile immobilization without the need for premodification of ligands or supports, and represents as a straightforward strategy for the rapid access of aryl-containing heterogeneous ligands or ligand/metal complexes. The resulting heterogeneous catalysts, Taniaphos/Cu(Ι) and Zhaophos/Rh(Ι), displayed excellent activity in asymmetric alkylation and hydrogenation reactions, achieving up to 99 % yield and 98 % enantiomeric excess (e.e.) respectively. Notably, the Zhaophos/Ir(Ι) heterogeneous catalyst exhibited more than double the performance in the asymmetric hydrogenation of benzoxazinones compared to its homogeneous counterpart. Moreover, all of the products could be obtained without chromatography purification with over 95 % NMR purity. Recycling tests and continuous flow experiments demonstrated that the heterogeneous catalyst could be reused for at least five times without any decrease in activity, or operated continuously for 100 h without loss of activity, achieving turnover numbers exceeding 1875 and reducing the reaction from 24 h to 4.7 min.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


