Ce3+, Eu2+-共掺杂Ca-α-Sialon荧光粉的能量转移实现广泛的颜色可调性和增强的热稳定性
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
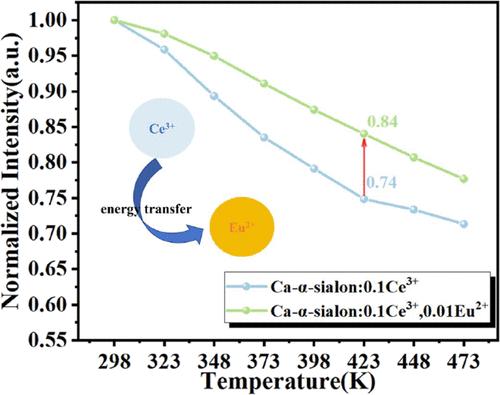
通过构建能量转移可以修饰和提高荧光粉的发光性能。本文采用传统的固相反应方法合成了一系列Ce3+-和/或Eu2+掺杂的Ca-α-硅酮荧光粉。高效能量传输的构建使调制范围广泛的光色成为可能,从而促进了从蓝绿(0.1844,0.2535)到黄(0.4282,0.5051)的渐变颜色变化。Ce3+的共掺杂使Eu2+的发光强度提高了约2.2倍。在实验梯度内,能量传递效率可达58.7%,能量传递机制为四极-四极相互作用。对共掺杂样品的发射光谱进行高斯拟合,结果表明,共掺杂样品在423 K时的Eu2+和Ce3+发射峰强度分别是室温时的78%和87%。此外,与单独掺杂相比,共掺杂样品中Ce3+特征峰的热稳定性显著增强。这些优异的性能突出了CaSi9Al3ON15/Ce3+, Eu2+荧光粉在白光wled中的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Realizing Broad Color Tunability and Enhanced Thermal Stability via Energy Transfer in Ce3+, Eu2+-Codoped Ca-α-Sialon Phosphors
Luminescent properties of phosphors can be modified and improved by constructing energy transfer. In this article, a series of Ce3+- and/or Eu2+-doped Ca-α-sialon phosphors were synthesized through the traditional solid-state reaction method. The construction of an efficient energy transfer enables the modulation of a wide range of light colors, thereby facilitating a gradual color change from blue-green (0.1844, 0.2535) to yellow (0.4282, 0.5051). Moreover, the codoping of Ce3+ enhances the luminescence intensity of Eu2+ by approximately 2.2 times. Within the experimental gradient, the energy-transfer efficiency can reach up to 58.7%, and the energy-transfer mechanism is a quadrupole–quadrupole interaction. Gaussian fitting of the emission spectrum of the codoped samples reveals that the intensities of the Eu2+ and Ce3+ emission peaks of the codoped samples at 423 K are 78 and 87% of those at room temperature, respectively. Moreover, the thermal stability of the characteristic peaks of Ce3+ in the codoped samples is significantly enhanced compared to those doped alone. These excellent properties highlight the potential application of CaSi9Al3ON15/Ce3+, Eu2+ phosphor in white light WLEDs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


