IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
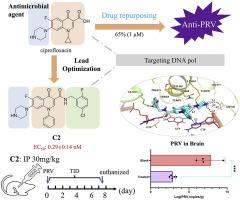
伪狂犬病毒(PRV)是影响猪健康的一种重要病原体,对人类构成很高的人畜共患病风险。针对 PRV 的有效抗病毒疗法仍然有限,这凸显了对新型治疗策略的需求。在本研究中,根据我们从 HSV-1 DNA 聚合酶抑制剂 PNU-183792 中得出的推断,喹诺酮类药物有可能成为抗 PRV 药物,因此环丙沙星被确定为有潜力的候选药物,在体外对 PRV 复制有显著抑制作用。化合物 A1 首先由环丙沙星和 PNU-183792 跳变而来,其抗 PRV 活性比环丙沙星高 500 多倍,EC50 为 2.21 nM。随后,通过快速优化,取代 A1 的苄基和环丙基,得到了 C2,该化合物对 PRV 复制有很好的抑制作用,EC50 为 0.29 nM,MIC80 为 1.6-8 nM。药代动力学研究表明,C2 具有良好的特性,腹腔给药后血浆暴露良好。昆明小鼠体内研究表明,C2 对 PRV 复制的抑制率高达 99%。这些结果表明,喹诺酮类抑制剂,尤其是 C2,是治疗 PRV 感染的一种可行疗法,值得进一步进行临床前开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Rapid discovery of pseudorabies virus inhibitors repurposed from the antimicrobial agent ciprofloxacin
Pseudorabies virus (PRV) is a significant pathogen impacting swine health and poses high zoonotic risks to humans. Effective antiviral treatments for PRV remain limited, underscoring the need for novel therapeutic strategies. In this study, ciprofloxacin was identified as a repurposed promising candidate with significant in vitro inhibition of PRV replication based on our inference from PNU-183792, an HSV-1 DNA polymerase inhibitor, that quinolones have the potential to act as anti-PRV drugs. Compound A1 was firstly hopping from ciprofloxacin and PNU-183792, showing more than 500-fold higher anti-PRV activity than ciprofloxacin with an EC50 of 2.21 nM. Then, C2 was obtained from rapid optimization of replacing the benzyl and cyclopropyl groups of A1, which showed excellent inhibition of PRV replication with an EC50 of 0.29 nM and MIC80 in range of 1.6–8 nM. Pharmacokinetic studies demonstrated favorable properties for C2, with good plasma exposure following intraperitoneal administration. In vivo studies in Kunming mice showed that C2 inhibited PRV replication by up to 99 %. These results suggest that quinolone-based inhibitors, particularly C2, represent a viable therapeutic approach for the treatment of PRV infections and warrant further preclinical development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


