类似马兰戈尼的组织流增强了胚胎器官组织的对称性突破
IF 18.4
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
摘要
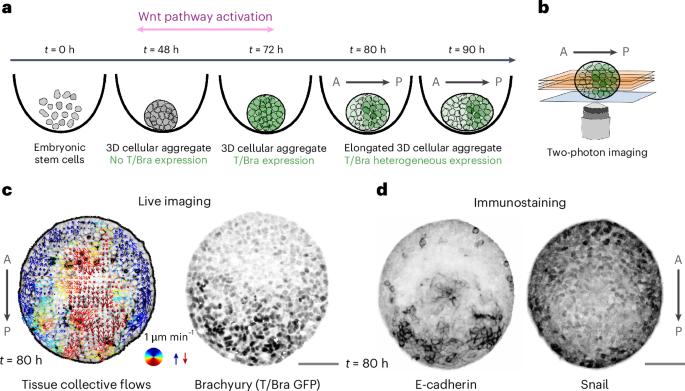
在多细胞动物的早期发育过程中,细胞自组织形成身体轴,如头尾轴。已知有几种信号通路控制体轴的形成。在这里,我们表明组织力学也起着重要作用。我们关注的是小鼠胚胎干细胞最初球形聚集体中主轴的出现,这反映了早期小鼠胚胎发育中的事件。这些聚集体通过建立不同表达谱的结构域来打破旋转对称性,例如转录因子T/Brachyury和粘附分子E-cadherin。通过结合定量显微镜和物理模型,我们确定了大规模的组织流动与再循环成分,这在很大程度上有助于对称性破坏。我们表明,再循环流动——类似于马兰戈尼流——是由组织表面张力的差异驱动的,我们通过聚集体融合实验进一步证实了其存在。我们的工作强调,身体轴的形成不仅是由生化过程驱动的,而且可以通过组织流动放大。我们期望这种类型的扩增可能在许多其他类器官和体内系统中起作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Marangoni-like tissue flows enhance symmetry breaking of embryonic organoids
During the early development of multi-cellular animals, cells self-organize to set up the body axes such as the primary head-to-tail axis. Several signalling pathways are known to control body axis formation. Here we show that tissue mechanics also plays an important role. We focus on the emergence of a primary axis in initially spherical aggregates of mouse embryonic stem cells, which mirrors events in the development of the early mouse embryo. These aggregates break rotational symmetry by establishing domains of different expression profiles, for example, of the transcription factor T/Brachyury and the adhesion molecule E-cadherin. By combining quantitative microscopy and physical modelling, we identify large-scale tissue flows with a recirculating component that contribute substantially to the symmetry breaking. We show that the recirculating flows are—akin to Marangoni flows—driven by a difference in tissue surface tensions, whose existence we further confirm using aggregate fusion experiments. Our work highlights that body axis formation is not only driven by biochemical processes but can also be amplified by tissue flows. We expect that this type of amplification may operate in many other organoid and in vivo systems. During the development of multi-cellular animals, biochemical signals control the organization of cells to set up body axes. In mouse embryonic stem cell aggregates, tissue flows are now found to amplify the formation of such body axes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


