催化结合质子萃取配体促进电催化CO2还原
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
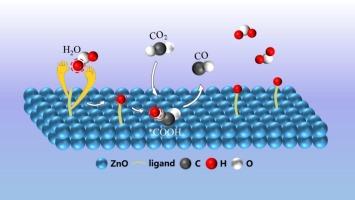
电化学CO2还原反应(eCO2RR)非常适合于降低大气CO2水平和产生清洁能源。水是电化学还原过程中的主要质子源。但由于水具有较高的解离能垒,且大部分质子都参与了HER反应,因此在CO2还原反应中只使用了少量的H+。本研究发现,在催化剂表面适当选择配体,可以从水中提取质子,将其储存并转移到CO2中,促进COOH的形成,提高eCO2RR的质子利用效率。此外,配体络合质子的活性低于自由质子的活性,从而导致HER抑制,我们使用不同pKa值(即质子释放量)的配体来功能化ZnO, pKa与FECO的曲线呈火山状。过小的pKa会导致丰富的质子相互结合和HER的优势,而过大的pKa则不会促进CO2对H+的利用。具有合适pKa的配体可以适当地结合和释放质子,有利于*COOH的形成,从而加速eCO2RR。特别是巯基苯甲酸(MBA)由于其适度的pKa (FECO = 90 %,比未改性ZnO的FECO高2倍,jCO的FECO高近5倍),显著提高了催化活性。原位红外光谱分析证明上述配体可以从H2O中提取质子并释放,促进H2O的解离。理论计算表明,pKa影响配体吸收和释放质子的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrocatalytic CO2 reduction promoted by catalyst-bound proton-abstracting ligands
The electrochemical CO2 reduction reaction (eCO2RR), which is well suited for reducing atmospheric CO2 levels and generating clean energy. Water is the main proton source in the electrochemical reduction process. However, water features a high dissociation energy barrier, and most protons are involved in HER, so that only a small amount of H+ is used in the CO2 reduction reaction. Herein, we reveal that appropriately chosen ligands on the catalyst surface can abstract protons from water, store them, and transfer them to CO2 to promote the formation of COOH and improve the proton utilization efficiency of the eCO2RR. Moreover, the activity of the ligand complexed protons is shown to be lower than that of free ones and thus result in HER inhibition our ligands with different pKa values (i.e., proton release capacities) are used to functionalize ZnO, and the plot of pKa vs. the FECO exhibits a volcano shape. Overly small pKa leads to the combination of abundant protons with each other and the dominance of the HER, whereas overly large pKa does not promote the utilization of H+ by CO2. Ligands with suitable pKa can properly bind and release protons, which is conducive to the formation of *COOH, thus accelerating eCO2RR. In particular, mercaptobenzoic acid (MBA) considerably enhances catalytic activity because of its moderate pKa (FECO = 90 %, which is 2 times higher than the FECO of unmodified ZnO, and jCO is nearly 5 times higher). In situ infrared spectroscopic analysis proves that the above ligand can abstract protons from H2O and then release them, promoting the dissociation of H2O. Theoretical calculations show that pKa affects the ability of ligands to abstract and release protons.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


