甲酸介导的转移氢化芳基卤化物交叉偶联
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
转移加氢反应在化学工业的各个领域都得到了广泛应用,但由于存在竞争性氢解反应,其在芳基卤化物还原交叉偶联反应中的应用尚未得到开发。本文利用 PdI 物种的独特反应活性,描述了一种高效催化系统,通过格式化介导的氢转移,将活化的芳基溴与芳基碘进行还原性交叉偶联。这些过程与铃木偶联和布赫瓦尔德-哈特维格偶联具有正交性,因为硼酸频哪醇酯和苯胺都能被容忍,而且由于螯合中间体的介入,对具有挑战性的 2-吡啶基体系也很有效。实验和计算研究证实了一种独特的还原交叉偶联催化循环,在这种循环中,PdI 前催化剂 [Pd(I)(PtBu3)]2 转化为二离子物种 [Pd2I4][NBu4]2,由此芳基卤化物的氧化加成更为容易。快速、可逆的 Pd 对 Pd 反金属化反应产生了碘桥式同二元和杂二元钯二聚体的混合物。杂二元钯二聚体更稳定,还原消除障碍更低,从而促进了高水平的交叉选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
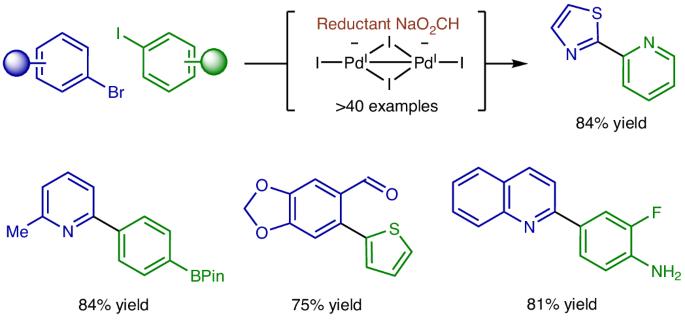
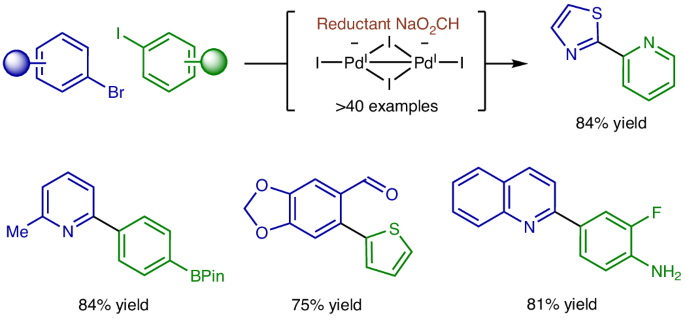
Aryl halide cross-coupling via formate-mediated transfer hydrogenation
Transfer hydrogenation is widely practised across all segments of chemical industry, yet its application to aryl halide reductive cross-coupling is undeveloped because of competing hydrogenolysis. Here, exploiting the distinct reactivity of PdI species, an efficient catalytic system for the reductive cross-coupling of activated aryl bromides with aryl iodides via formate-mediated hydrogen transfer is described. These processes display orthogonality with respect to Suzuki and Buchwald–Hartwig couplings, as pinacol boronates and anilines are tolerated and, owing to the intervention of chelated intermediates, are effective for challenging 2-pyridyl systems. Experimental and computational studies corroborate a unique catalytic cycle for reductive cross-coupling where the PdI precatalyst, [Pd(I)(PtBu3)]2, is converted to the dianionic species, [Pd2I4][NBu4]2, from which aryl halide oxidative addition is more facile. Rapid, reversible Pd-to-Pd transmetallation delivers mixtures of iodide-bridged homo- and hetero-diarylpalladium dimers. The hetero-diarylpalladium dimers are more stable and have lower barriers to reductive elimination, promoting high levels of cross-selectivity. Transfer hydrogenation is challenging to apply to aryl halide reductive cross-couplings because of competing hydrogenolysis. Now aryl halide cross-couplings mediated by sodium formate have been developed. These processes display orthogonality to Suzuki and Buchwald–Hartwig couplings as pinacol boronates and anilines are tolerated and, owing to chelated intermediates, effective for challenging 2-pyridyl systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


