具有特殊容量的高可充电水性锌对苯二酚电池的微孔限制。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
报道了一种利用锌阳极、硫酸锌水溶液电解质和对苯二酚(QH2)阴极的高可充电电池。固定在微孔碳孔内的QH2具有较高的比容量(0.5℃时为482 mA h g-1),接近QH2的理论比容量(486.8 mA h g-1)。即使在1000次充放电循环后仍保持高容量(99%保留初始充电容量),库仑效率为99%。绿色环保的锌- qh2电池不含重金属或过渡金属离子,不含腐蚀性或易燃性电解质,利用了丰富易得的材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
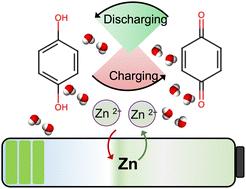
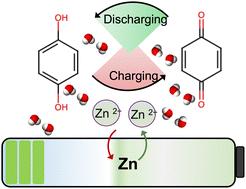
Micropore confinement for a highly rechargeable aqueous Zn-hydroquinone battery with exceptional capacity†
A highly rechargeable battery utilizing a zinc anode, aqueous ZnSO4 electrolyte, and hydroquinone (QH2) cathode is reported. QH2 immobilized within the pores of microporous carbon delivered a high specific capacity (482 mA h g−1 at 0.5C), approaching the theoretical specific capacity of QH2 (486.8 mA h g−1). A high capacity was maintained even after 1000 charge–discharge cycles (99% retention of initial charge capacity), with 99% coulombic efficiency. The environentally green Zn–QH2 battery did not include any heavy or transition metal ions, or corrosive or flammable electrolytes, and utilized abundant and readily available materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


