含密集叔丁基侧链类肽低聚物的固相合成
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
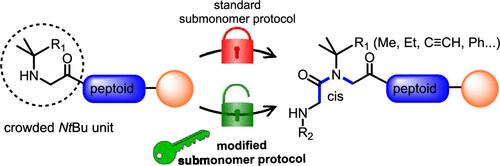
N- 叔丁基甘氨酸(NtBu)是一种已知的类肽结构诱导单体。受阻的叔丁基对 NX-NtBu 蛋白胨-酰胺键的顺式/反式异构化具有重要影响,该键只采用顺式几何结构。因此,在蛋白胨低聚物中加入这种单体是促进特定二级结构(如转折和多脯氨酸 I 型螺旋)的绝佳方法。然而,叔丁基的立体阻碍迄今为止一直阻碍着含有 NtBu 单体的类胨低聚物的固相合成。我们在此首次报告了使用改进的亚单体方案固相合成含 NtBu 的蛋白胨的情况。我们发现,DIC 介导的关键酰化步骤能否成功取决于反应前是否添加碱和/或对树脂进行碱性预处理。使用氯乙酸代替溴乙酸以及卤代乙酸预活化阶段也能提高合成效率。为了证明改良亚单体方案的有效性,我们合成了序列长度达 12 个单体的均寡聚体,并将其用于合成具有高度拥挤的 NCα-gem 二甲基侧链的各种拟肽类化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Solid-Phase Synthesis of Peptoid Oligomers Containing Crowded tert-Butyl Side Chains
N-tert-butyl-glycine (NtBu) is a known peptoid structure-inducing monomer. The hindered tert-butyl group exerts a major effect on the cis/trans isomerization of the NX-NtBu peptoid-amide bond, which adopts exclusively the cis-geometry. Incorporating this monomer into peptoid oligomers is therefore an excellent way of promoting specific secondary structures such as turns and polyproline type-I helices. However, the steric hindrance of the tert-butyl group has so far prevented the solid-phase synthesis of peptoid oligomers incorporating NtBu monomers. We report here for the first time solid-phase syntheses of NtBu-containing peptoids using a modified submonomer protocol. We have found that the success of the critical DIC-mediated acylation step depends on the addition of a base and/or basic pretreatment of the resin prior to the reaction. The use of chloroacetic acid instead of bromoacetic acid also improved the efficacy of the syntheses, as did a halogenoacetic acid preactivation stage. To demonstrate the effectiveness of the modified submonomer protocol, we synthesized homooligomers at sequence lengths of up to 12-mer and also applied it to the synthesis of various peptoids with highly congested NCα-gem-dimethyl side chains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


