集成微流控芯片用于中性粒细胞胞外泡分析和胃癌诊断
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
中性粒细胞衍生的细胞外囊泡(nev)在疾病进展中起着至关重要的作用,被认为是潜在的生物标志物。然而,新能源汽车分离和检测过程繁琐,限制了新能源汽车的使用。在此,我们提出了一种用于NEV分析的集成微流控芯片(IMCN),该芯片实现了CD66b+ NEV的免疫分离,并通过10 μL血清样本对其所含的mirna(称为NEV特征)进行多路检测。优化的微通道和IMCN芯片的流量使新能源汽车的有效捕获(>90%)。在捕获的新能源汽车被特定的CD63适配体识别后,热裂解的新能源汽车释放的适配体和mirna触发片上滚动圈扩增(RCA)反应。然后,RCA产物结合到分子信标(mb)上,启动变构发夹结构并放大“打开”荧光信号(RCA- mb测定)。临床样本分析表明,NEV特征在健康对照(HC)与胃癌(GC)、良性胃病(BGD)与胃癌(0.857)之间具有较高的曲线下面积(AUC)。值得注意的是,结合5种生物标志物(NEV特征、CEA和CA199)区分GC和HC的AUC达到0.912,并且使用基于机器学习(ML)的集成分类系统进一步提高了诊断准确率。因此,所开发的IMCN芯片是一个有价值的NEV分析平台,可能在GC诊断中具有潜在的应用价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
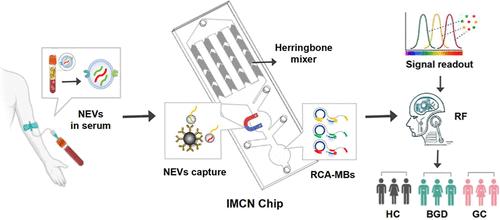
Integrated Microfluidic Chip for Neutrophil Extracellular Vesicle Analysis and Gastric Cancer Diagnosis
Neutrophil-derived extracellular vesicles (NEVs) are critically involved in disease progression and are considered potential biomarkers. However, the tedious processes of NEV separation and detection restrain their use. Herein, we presented an integrated microfluidic chip for NEV (IMCN) analysis, which achieved immune-separation of CD66b+ NEVs and multiplexed detection of their contained miRNAs (termed NEV signatures) by using 10 μL serum samples. The optimized microchannel and flow rate of the IMCN chip enabled efficient capture of NEVs (>90%). After recognition of the captured NEVs by a specific CD63 aptamer, on-chip rolling circle amplification (RCA) reaction was triggered by the released aptamers and miRNAs from heat-lysed NEVs. Then, the RCA products bound to molecular beacons (MBs), initiating allosteric hairpin structures and amplified “turn on” fluorescence signals (RCA-MB assay). Clinical sample analysis showed that NEV signatures had a high area under curve (AUC) in distinguishing between healthy control (HC) and gastric cancer (GC) (0.891), benign gastric diseases (BGD) and GC (0.857). Notably, the AUC reached 0.912 with a combination of five biomarkers (NEV signatures, CEA, and CA199) to differentiate GC from HC, and the diagnostic accuracy was further increased by using a machine learning (ML)-based ensemble classification system. Therefore, the developed IMCN chip is a valuable platform for NEV analysis and may have potential use in GC diagnosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


