用于环氧/胺热固性树脂的香草醇和香草醇/木质素磺酸混合物自酸缩合的可再生酚醛低聚物
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
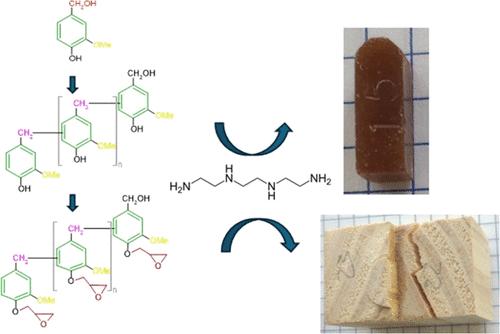
从双酚中提取的环氧树脂来源于化石燃料,因其干扰内分泌的特性而引起健康问题,因此需要可再生且毒性较低的替代品。木质素及其衍生物为双酚提供了潜在的替代品,尽管目前的解决方案面临着局限性。在这里,使用木质素衍生前体进行的酸缩合反应为环氧树脂/胺热固性塑料中的双酚提供了潜在的替代品。在回流条件下,所选前体香草醇和甘蔗木质素磺酸盐溶于水,确保了水相的均匀性和反应的快速性。香草醇的自缩合产生了聚合度为 2-6 的不溶性酚类低聚物。在反应中加入木质素磺酸盐会产生摩尔质量更高的产品,因为木质素磺酸盐的含量为 21%(重量比)。二维-HSQC NMR 分析深入揭示了通过苄醇脱水,然后在芳香环的 C5 和 C6 位置发生主要缩合的低聚过程。由此产生的酚醛低聚物可生成油性质地的环氧化树脂,即使加入木质素磺酸盐也是如此,并且适合与多胺一起固化。生产出的热固性树脂具有相对较高的热稳定性和高效的木材粘合性能。新开发的生物质衍生环氧树脂可作为双酚的可行替代品,具有潜在的环境效益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Renewable Phenolic Oligomers from Self-Acid Condensation of Vanillyl Alcohol and Vanillyl Alcohol/Lignosulfonate Mixtures for Use in Epoxy/Amine Thermosets
Epoxy resins derived from bisphenol are sourced from fossil fuels and are linked to health concerns due to their endocrine-disrupting properties, highlighting the need for renewable and less toxic alternatives. Lignin and its derivatives offer potential replacements for bisphenol, although the current solutions face limitations. Here, acid condensation reactions using lignin-derived precursors provided potential substitutes for bisphenol in epoxy/amine thermosets. Under reflux, the selected precursors, vanillyl alcohol and sugarcane lignosulfonate, dissolved in water, ensuring a homogeneous aqueous phase and rapid reaction. The self-condensation of vanillyl alcohol produced insoluble phenolic oligomers with a degree of polymerization of 2–6. Incorporating lignosulfonate into the reaction resulted in higher-molar-mass products due to 21% (w/w) lignosulfonate integration. 2D-HSQC NMR analysis provided insights into the oligomerization process that occurred via benzylic alcohol dehydration followed by predominant condensation at the C5 and C6 positions of the aromatic ring. The resulting phenolic oligomers produced oily textured epoxidated resins, even with lignosulfonate incorporation, and were suitable for curing with polyamines. Produced thermosets presented relatively high thermal stability and efficient wood adhesive properties. The newly developed biomass-derived epoxy resins could serve as viable substitutes for bisphenol with potential environmental benefits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


