IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
设计用于从 H2 和 O2 直接合成过氧化氢(DSHP)的高效钯基催化剂仍然是一项挑战。本文通过在 N2 气氛下热解 ZIF-8 合成了掺杂 Zn 的多孔碳,并将其用作 DSHP 的 Pd 催化剂载体。畸变校正 HAADF-STEM、XRD、XPS 和 TEM 的结果表明,多孔碳载体中的 Zn 物种主要存在于高度分散的 ZnNx 中。在还原过程中,Zn 原子首先被相邻 Pd0 原子溢出的氢还原,然后扩散到 Pd 颗粒中形成金属间 PdZn。热解温度似乎是影响 PdZn 比例的关键因素。热解温度为 850 ℃ 时获得的多孔碳支撑催化剂中 PdZn 的比例最高(Pd/ZnC-850)。DFT 模拟显示,金属间 PdZn 使 Pd 原子的 d 带中心偏离费米级;减弱了对 O2、*OOH 和 H2O2 的吸附;缩短了*OOH 和 H2O2 的 O-O 键长度。这些都有利于抑制 O-O 键的裂解。Pd/ZnC-850 具有出色的 H2O2 选择性(89.6%)、相对较高的 H2 转化率和良好的稳定性。H2O2 生产率达到 44,396 mol kgPd-1 h-1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
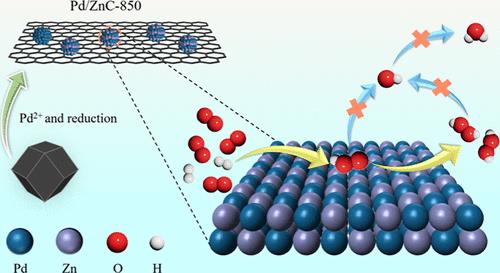
Intermetallic PdZn Supported on Porous Carbon Derived from ZIF-8 for Efficient Direct Synthesis of H2O2
Design of highly efficient Pd-based catalysts for the direct synthesis of hydrogen peroxide (DSHP) from H2 and O2 still remains a challenge. Herein, Zn-doped porous carbon was synthesized via pyrolysis of ZIF-8 under a N2 atmosphere and then applied as supports of Pd catalysts for DSHP. The results of aberration corrected HAADF-STEM, XRD, XPS, and TEM reveal that the Zn species in the porous carbon support exist mainly in highly dispersed ZnNx. In the reduction process, Zn atoms are first reduced by spillover hydrogen from adjacent Pd0 atoms and then diffuse into Pd particles to form intermetallic PdZn. The pyrolysis temperature appears to be a crucial factor affecting the proportion of PdZn. A catalyst supported on the porous carbon obtained at a pyrolysis temperature of 850 °C has the highest proportion of PdZn (Pd/ZnC-850). The DFT simulations reveal that intermetallic PdZn shifts the d-band center of Pd atoms away from the Fermi level; weakens the adsorption of O2, *OOH, and H2O2; and shortens the length of the O–O bonds of *OOH and H2O2. These are favorable for inhibiting cleavage of O–O bonds. Pd/ZnC-850 exhibits a superior H2O2 selectivity (89.6%), relatively high H2 conversion, and good stability. A H2O2 productivity of 44,396 mol kgPd–1 h–1 is achieved.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


