扩展π共轭的吲哚菁多甲基荧光团,发光超过1200 nm,用于增强NIR-II成像
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
吲哚菁聚甲胺是临床上最有前景的荧光成像探针之一。波长更长的近红外-II 探针可提高组织穿透深度和信噪比(SNR),从而增强荧光成像性能,但这往往会导致亮度降低。尽管多次尝试对吲哚菁多甲胺的波长进行红移,但其发射波长仍局限在 1,103 nm。我们报告了第一种发射波长超过 1,200 nm 的吲哚菁多甲胺 Cy15s,其最长最大发射峰值为 1,287 nm,同时在二氯乙烷(DCM)中保持 117.1 M-1⋅cm-1 的高亮度,是发射波长超过 1,200 nm 的多甲胺荧光团最佳性能的 6 倍。低细胞毒性和显著的光学特性使得高质量的近红外 II (NIR-II)b 血管造影和长期正位肿瘤成像成为可能。除了相对成熟的聚甲胺末端基团研究外,本研究还引入了一种新型共轭链支架,为设计和合成用于深部组织成像和肿瘤研究的近红外 II 探针开辟了新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
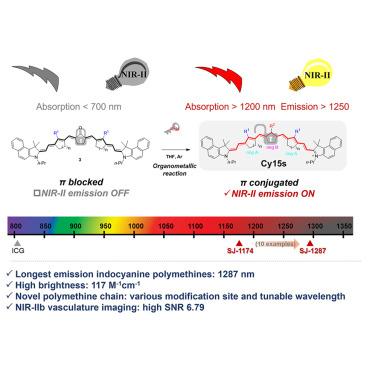
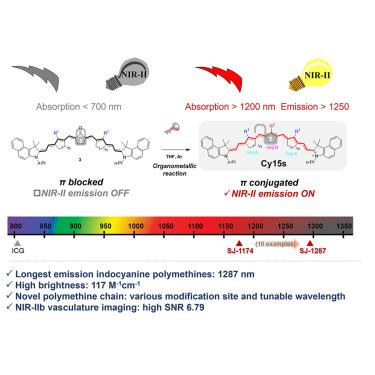
Indocyanine polymethine fluorophores with extended π-conjugation emitting beyond 1,200 nm for enhanced NIR-II imaging
Indocyanine polymethines are among the most clinically promising probes for fluorescence imaging. Longer wavelength NIR-II probes offer enhanced fluorescence imaging performance by improving the tissue penetration depth and signal-to-noise ratio (SNR), but this often results in reduced brightness. Despite multiple attempts to redshift indocyanine polymethines' wavelengths, their emission wavelengths are restricted to 1,103 nm. We report the first indocyanine polymethines, Cy15s, emitting beyond 1,200 nm, with the longest maximum peak emission at 1,287 nm while maintaining a high brightness of 117.1 M−1⋅cm−1 in dichloroethane (DCM), 6-fold of the best performance of polymethine fluorophores emitting over 1,200 nm. The low cytotoxicity and remarkable optical properties enable high-quality near-infrared II (NIR-II)b angiography and long-term orthotopic tumor imaging. In addition to the relatively mature terminal groups research of polymethines, this study introduces a novel scaffold for conjugation chains, opening new avenues for the design and synthesis of NIR-II probes for deep-tissue imaging and tumor research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


