携带 Tenascin-C 的细胞外小泡是临床生物标记物,可改善胶质母细胞瘤患者的肿瘤衍生 DNA 分析
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
细胞外囊泡(EVs)作为从肿瘤到血液的生物信息的载体,能够检测循环肿瘤物质并跟踪疾病进展。这在胶质母细胞瘤中尤其重要,胶质母细胞瘤是一种高度侵袭性和异质性的肿瘤,很难监测。利用成像流式细胞术(IFCM),我们对37例新诊断的胶质母细胞瘤患者(手术前和手术后)、11例复发性胶质母细胞瘤患者和22例健康供体(HD)的血浆EVs中8种胶质瘤相关抗原和四联蛋白进行了免疫表型分析。与非肿瘤患者相比,Tenascin-C (TNC)阳性ev在新诊断和复发的胶质母细胞瘤患者中表现出最大的差异。在双阳性亚群中,TNC+/CD9+ EVs在新诊断患者(FC = 7.6, p <0.0001, AUC = 81%)和复发患者(FC = 16.5, p <0.0001;AUC = 90%)高于HD。与其他中枢神经系统肿瘤(n = 25)相比,胶质母细胞瘤的这一亚群也比脑膜瘤高34.5倍(p <0.01)。此外,胶质母细胞瘤患者(n = 6)脑脊液中TNC+/CD9+ EV水平比对照组高3.3倍(p <0.05)。在不同来源的胶质母细胞瘤ev中进一步观察到异常的TNC水平,并通过不同的方法纯化。免疫组化分析显示肿瘤组织中TNC含量高。空间转录组学分析表明,TNC在胶质母细胞瘤切除的恶性细胞群中过表达,特别是在具有间充质样特征和染色体畸变的细胞中。最后,采用磁分选法从21例胶质母细胞瘤患者血浆中纯化出TNC+ ev,并采用液滴数字PCR检测致癌突变TERT*C228T。突变等位基因频率在TNC阳性EVs中高于TNC阴性EVs (FC = 32, p <0.001)、总EVs (FC = 5.3, p <0.001)或无细胞DNA (FC = 5.3, p <0.01)。综上所述,循环TNC+ ev可能有潜力作为胶质母细胞瘤的临床生物标志物,它们的纯化可以提高液体活检中肿瘤特异性突变的识别。本文章由计算机程序翻译,如有差异,请以英文原文为准。
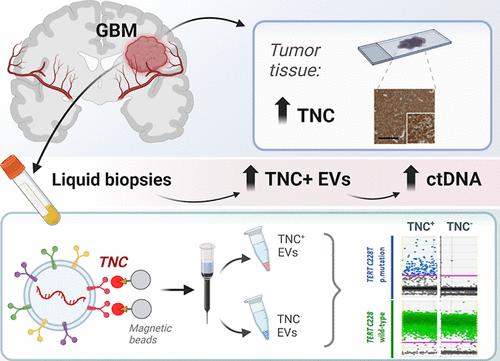
Extracellular Vesicles Carrying Tenascin-C are Clinical Biomarkers and Improve Tumor-Derived DNA Analysis in Glioblastoma Patients
Extracellular vesicles (EVs) act as carriers of biological information from tumors to the bloodstream, enabling the detection of circulating tumor material and tracking of disease progression. This is particularly crucial in glioblastoma, a highly aggressive and heterogeneous tumor that is challenging to monitor. Using imaging flow cytometry (IFCM), we conducted an immunophenotyping analysis of eight glioma-associated antigens and tetraspanins in plasma EVs from 37 newly diagnosed glioblastoma patients (pre- and post-surgery), 11 matched individuals with recurrent glioblastoma, and 22 healthy donors (HD). Tenascin-C (TNC) positive EVs displayed the strongest differences in newly diagnosed and recurrent glioblastoma patients, when compared to non-tumor subjects. Among dual-positive subpopulations, TNC+/CD9+ EVs were the most elevated in newly diagnosed (FC = 7.6, p <0.0001, AUC = 81%) and recurrent patients (FC = 16.5, p <0.0001; AUC = 90%) than HD. In comparison with other CNS tumors (n = 25), this subpopulation was also 34.5-fold higher in glioblastoma than in meningioma cases (p <0.01). Additionally, TNC+/CD9+ EV levels were 3.3-fold elevated in cerebrospinal fluid from glioblastoma patients (n = 6) than controls (p <0.05). Aberrant TNC levels were further observed in glioblastoma EVs from different sources and purified via different methods. Immunohistochemical analysis revealed high levels of TNC in tumor tissues. Spatial transcriptomic analysis indicated a TNC overexpression in malignant cell populations of glioblastoma resections, particularly in cells with mesenchymal-like signatures and chromosomal aberrations. Lastly, we purified TNC+ EVs from plasma of 21 glioblastoma patients by magnetic sorting and detected the oncogenic mutation TERT*C228T by droplet digital PCR. The mutant allele frequency was higher in TNC+ EVs vs TNC-negative EVs (FC = 32, p <0.001), total EVs (FC = 5.3, p <0.001) or cell-free DNA (FC = 5.3, p <0.01). In conclusion, circulating TNC+ EVs may have potential as clinical biomarkers in glioblastoma, and their purification could improve the identification of tumor-specific mutations in liquid biopsies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


