弱溶剂化电解质对层状富锂氧化物界面化学的调控
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
用于锂离子电池的层状富锂氧化物(LLRO)阴极具有出色的比容量,但存在不可逆的氧释放,导致电压持续衰减。电解质与正极材料之间的界面化学对提高LLRO的稳定性至关重要。商业碳酸盐电解质(CCE)倾向于形成富有机物的界面相,在循环过程中易溶解。相反,富无机间相是坚固的和电化学稳定的。为了实现这一目标,使用二氟草酸硼酸锂(LiDFOB)和三氟(2,2,2-三氟乙基)磷酸(TFEP)配制弱溶剂化电解质(WSE)。在本设计中,DFOB阴离子参与初级溶剂化鞘层(PSS),形成阴离子为主的结构,促进了无机富锂基阴极电解质界面(CEI)的形成。电化学性能表明,WSE能显著抑制电压衰减,平均电压降仅为0.45 mV/循环,而CCE的平均电压降为2.51 mV/循环。阴离子衍生的间相减缓了LLRO的失效过程,为提高材料稳定性的电解质设计提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
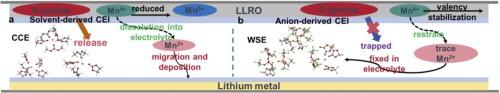
Regulating interfacial chemistry of layered lithium-rich oxide by weakly solvating electrolyte
Layered lithium-rich oxide (LLRO) cathodes for lithium-ion batteries exhibit outstanding specific capacity but suffer from irreversible oxygen release, which leads to continuous voltage decay. The interfacial chemistry between electrolyte and cathode material is crucial for improving the stability of LLRO. Commercial carbonate electrolytes (CCE) tend to form an organic-rich interphase, which is susceptible to dissolution during cycling. On the contrary, an inorganic-rich interphase is robust and electrochemically stable. To achieve this goal, lithium difluoro-oxalate borate (LiDFOB) and tris(2,2,2-trifluoroethyl) phosphate (TFEP) are used to formulate a weakly solvating electrolyte (WSE). In this design, DFOB- anion participates in the primary solvation sheath (PSS), forming an anion-dominated structure that facilitates the formation of an inorganic-rich, LiF-based cathode electrolyte interphase (CEI). Electrochemical performance indicates that voltage decay is significantly suppressed in WSE, with an average voltage drop of only 0.45 mV/cycle, compared to 2.51 mV/cycle in CCE. The anion-derived interphase slows the failure process of LLRO, providing valuable insights into electrolyte design for enhancing material stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


