集合Lewis位萃取材料和串联电化学装置直接回收含氟核废水中的高纯铀
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
从含高浓度氟离子(F-)的真实核废水中电化学提铀是一种有效处理放射性废水和回收宝贵铀资源的有前途的策略。然而,目前的进展受到F-浓度极高和最终铀产品纯度不理想的干扰。在此,我们构建了铋氧化物中相邻的系综Lewis酸碱对位点(系综Lewis位点)作为萃取材料,并将其集成到设计的串联电化学装置中,用于从实际核废水中高效回收高纯度铀。机制研究表明,集合Lewis位点通过同时加强与U、O和F原子的化学键,显著增强了所有优势氟化铀酰(UO2Fx)的结合。串联电化学装置合理控制了U3O8的反应周期,避免了向K2U2O7的结晶转变。在1 L真实核废水中电解3 h,铀的提取率达到99.9%,提取的铀产物中碱金属杂质仅为2.2%,优于以往的工作。本研究为实际核废水中铀资源的回收提供了一种有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
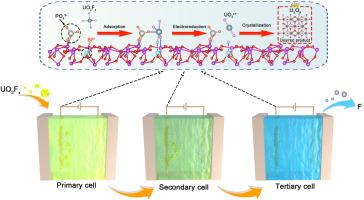
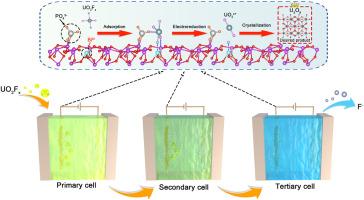
Direct recovery of high-purity uranium from fluoride-containing nuclear wastewater via extraction materials with ensemble Lewis sites and a tandem electrochemical device
Electrochemical uranium extraction from real nuclear wastewater with a high concentration of fluoride ions (F-) represents a promising strategy for the efficient treatment of radioactive wastewater and the recovery of the valuable uranium resource. However, the current progress suffers from the interference of extremely high concentration of F- and undesired purity of the final uranium product. Herein, we constructed the neighboring ensemble Lewis acid-base pair sites (ensemble Lewis sites) in bismuth oxides as the extraction material, which was integrated into a designed tandem electrochemical device for efficient recovery of high-purity uranium from real nuclear wastewater. The mechanistic study revealed that the ensemble Lewis sites dramatically enhance the binding of all dominant uranyl fluoride (UO2Fx) species through the simultaneous strengthened chemical bonds with U, O, and F atoms. Besides, the tandem electrochemical device rationally controlled the reaction period to U3O8, avoiding the undesired crystalline transformation to K2U2O7. Through 3 h electrolysis in 1 L of real nuclear wastewater, the extraction efficiency of uranium reached 99.9 % with only 2.2 % impurities of alkali metals in extracted uranium product, outperforming the previous work. This study offers an effective method for the recovery of uranium resources in real nuclear wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


