C4吲哚戊烯基转移酶FgaPT2的理论研究和突变实验
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
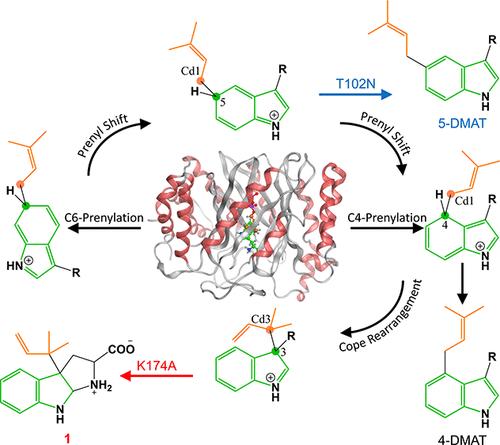
吲哚戊烯基转移酶(IPTs)在丰富的天然产物的生物合成中发挥着重要作用。然而,具体成员的潜在机制尚不完全清楚。本文通过多尺度计算和实验验证,研究了参与麦角生物碱重要药物合成的C4 IPT FgaPT2的详细反应机理。我们的研究表明,FgaPT2中的c4 -戊烯酰化过程是一种非常规的结合反应,伴随着短暂的碳正离子中间体,而不是解离反应。FgaPT2中的酪氨酸屏蔽主要通过与二磷酸二甲基烯丙基的氢键相互作用促进戊烯酰化步骤。保守的E89残基通过静电相互作用显著降低了戊酰化步骤的能垒。我们还在K174A突变体中证实了Cope重排过程,这导致了一个反向戊烯化的C3三环产物。通过对MD轨迹的分析,我们提出了C6-戊烯基化机制和从C6到C5,最后到C4位点的戊烯基移位反应,并通过QM计算和QCT-MD模拟进行了识别。基于我们提出的新机制,通过合理的工程设计,成功地在T102N突变体中获得了c5 -戊烯基化产物5-二甲基烯丙基色氨酸。我们的研究扩展了目前对IPTs催化机理的理解,并为合理修饰IPTs以合成各种高价值的烯丙基化吲哚产品提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Understanding and Engineering of C4 Indole Prenyltransferase FgaPT2 by Theoretical Study and Mutation Experiments
Indole prenyltransferases (IPTs) play vital roles in the biosynthesis of abundant natural products with diverse biological activities. However, the underlying mechanisms of specific members are not fully understood. Herein, we investigated the detailed reaction mechanism of FgaPT2, a C4 IPT involved in the biosynthesis of important pharmaceutical ergot alkaloids, by employing multiscale calculations and experimental validation. Our study indicates that the C4-prenylation process in FgaPT2 is an unconventional associative reaction accompanied by a short-lived carbocation intermediate rather than a dissociative reaction. The tyrosine shield in FgaPT2 facilitates the prenylation step mainly through hydrogen bond interactions with the dimethylallyl diphosphate. The conserved E89 residue significantly lowers the energy barrier of the prenylation step through the electrostatic interaction. We also confirmed the Cope rearrangement process in the K174A mutant, which results in a reverse-prenylated C3 tricyclic product. By analyzing the MD trajectories, we propose a C6-prenylation mechanism and a prenyl shift reaction from C6 to C5, and finally to the C4 site, which was identified by QM calculation and QCT-MD simulation. Based on our newly proposed mechanism, the C5-prenylated product 5-dimethylallyltryptophan was successfully obtained in the T102N mutant through rational engineering. Our study expands the current understanding of the catalytic mechanism of IPTs and provides insights into the rational modification of IPTs to synthesize a wide variety of high-value prenylated indole products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


