Ni(II)配位球内阻碍异氰化物停止聚合的快照
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
含有二烷基二硫代磷酸盐配体的阳离子Ni(II)配合物与位阻异氰酸酯结合使用抑制聚合,允许形成定义良好的单体阳离子Ni(II)配合物3,这是由三种异氰酸酯偶联产生的。这些配合物已经被表征,包括x射线结构测定,并代表了异氰化物聚合的第一步的快照。通过x射线、红外和核磁共振的研究似乎表明,在Ni(II)催化的异氰化物聚合及其所谓的“旋转木马”机制中,关键的活性物质并不是像之前提出的那样是一种碳烯,而是一种甲脒基物质。利用空间拥塞度最高的配体,分离出仅含有两个偶联异氰酸酯的中间产物4c,可用于利用两种不同异氰酸酯逐步可控合成稀有的混合Ni(II)配合物5。本文章由计算机程序翻译,如有差异,请以英文原文为准。
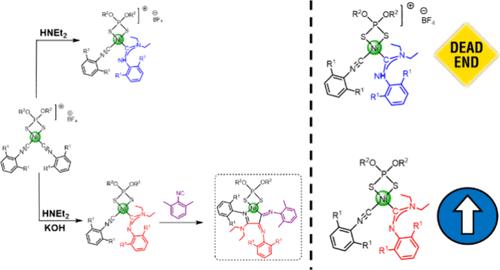
Snapshots of the Stopped Polymerization of a Hindered Isocyanide within the Coordination Sphere of Ni(II)
The use of cationic Ni(II) complexes containing dialkyldithiophosphate ligands in conjunction with sterically hindered isocyanides suppresses polymerization, allowing the formation of well-defined monomeric cationic Ni(II) complexes 3 that result from the coupling of three isocyanides. These complexes have been characterized, including X-ray structure determination, and represent a snapshot of the first steps of the polymerization of isocyanide. Studies via X-ray, IR, and NMR seem to indicate that the key active species in the Ni(II)-catalyzed isocyanide polymerization and its so-called “merry-go-round” mechanism is not a carbene, as has been proposed, but actually a formamidinyl species. The use of the most sterically congested set of ligands enabled the isolation of the intermediate species 4c, which contains only two coupled isocyanides and can be used in the stepwise and controlled synthesis of a rare mixed Ni(II) complex 5 by using two different isocyanides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


