无催化剂、可扩展、绿光介导的碘胺化和烯烃在连续流动条件下的进一步转化
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
我们报道了在无金属、可见光介导的条件下,用市售的n -碘琥珀酰亚胺和保护胺在连续流中对烯烃进行碘胺化。未活化和活化的烯烃以及迈克尔受体都是该工艺的底物类别,可以合成范围广,收率高(59-94%)的1,2-碘胺。在4.5 h的连续流动实验中,对苯乙烯、NIS和N-tosyl-amine偶联得到了14 g(91%)的碘化产物,相当于3.1 g h - 1的生产率,证明了该方案的稳定性。此外,无需事先分离的产品直接转化为叠氮嘧啶、胺类、氨基醇或叠氮多胺是可能的,强调了这种方法的合成价值。通过在试剂或溶剂中添加典型杂质或将照射从绿光改为蓝光来改变反应条件,对收率的影响很小,这表明该过程具有稳健性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
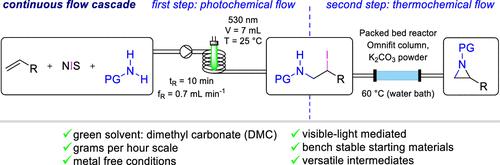
Catalyst-Free, Scalable, Green-Light-Mediated Iodoamination, and Further Transformation of Olefins Under Continuous Flow Conditions
We report the iodoamination of alkenes in continuous flow under metal-free, visible-light-mediated conditions with commercially available N-iodosuccinimide and protected amines. Unactivated and activated alkenes as well as Michael acceptors are amenable substrate classes for this process, allowing the synthesis of 1,2-iodoamines with a broad scope and in high yields (59–94%). The steadiness of the protocol is demonstrated in a continuous flow experiment over 4.5 h for the coupling of styrene, NIS, and N-tosyl-amine, which gave rise to 14 g (91%) of the iodoaminated product, corresponding to a productivity of 3.1 g h–1. Additionally, the direct conversion of the products without prior isolation into aziridines, enamines, amino alcohols, or azidoamines is possible, underscoring the synthetic value of this approach. Variation of the reaction conditions by adding typical impurities in reagents or solvents or changing the irradiation from green to blue light had a minimal effect on the yield, giving credit to the robustness of the process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


