用于尿路感染快速精确抗生素处方指南的自校准化学发光传感器
IF 9.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
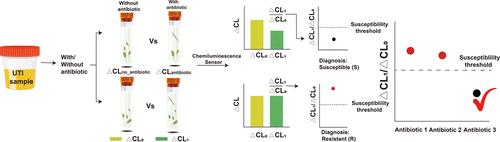
传统的抗菌素敏感性检测方法,包括从尿液样本中分离和培养细菌以及抗生素敏感性试验(AST),既昂贵又耗时。因此,迫切需要一种快速、用户友好的AST表型来指导治疗策略。基于直接从临床尿液样本中获得的细菌生理特征的几种新型表型AST平台已被提出作为快速AST和适当抗生素治疗的有前途的方法。然而,当需要使用这些方法进行高精度定量分析时,不准确的细菌定量可能导致错误的结果。将病原体过氧化氢酶的表达与基于化学发光的分析方法结合起来,可以实现方便和低成本的操作。在此,我们展示了一种快速自校准的化学发光传感器,可以通过过氧化氢酶活性的变化及其对过氧化氢的反应来测量细菌的活力。该快速纳米传感器平台可在40分钟内直接从临床尿液样本中检测尿路致病性大肠杆菌和肺炎克雷伯菌的抗生素敏感性,这两种细菌占所有尿路感染的80%,无需细菌定量。所提出的超快速、高精度AST可以实现抗生素处方的精确指导,缩短临床决策所需的时间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Self-Calibrating Chemiluminescence Sensor for Rapid and Precise Antibiotic Prescribing Guidelines on Urinary Tract Infections
Traditional antimicrobial-susceptibility testing methodologies, including the isolation and culture of bacteria from urine samples and antibiotic-susceptibility test (AST), are expensive and time-consuming. Therefore, a rapid, user-friendly phenotypic AST is urgently needed to guide treatment strategies. Several novel phenotypic AST platforms based on the physiological characteristics of bacteria obtained directly from clinical urine samples have been proposed as promising methods as rapid AST and appropriate antibiotic treatments. However, inaccurate bacterial quantification can lead to false results when high-accuracy quantitative assays are required using these procedures. Coupling the expression of catalase by pathogens with a chemiluminescence-based analytical method enables a convenient and low-cost operation. Herein, we demonstrate a rapid self-calibrating chemiluminescence sensor that can measure bacterial viability through the variation in catalase activity and its response to hydrogen peroxide after treatment with antibiotics. This rapid nanosensor platform can be utilized to determine the antibiotic susceptibility of uropathogenic Escherichia coli and Klebsiella pneumoniae, which account for 80% of all urinary tract infections, directly from clinical urine samples within 40 min without bacterial quantification. The proposed ultrafast and highly accurate AST can enable the precise guidance of antibiotic prescriptions and shorten the time required for clinical decision-making.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


