可调整合素-配体耦合强度调节细胞自适应机械传感
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
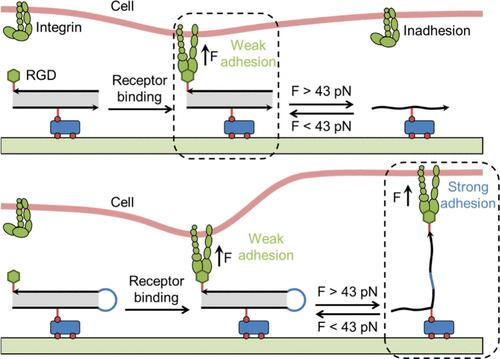
细胞通过整合素与整合素特异性配体的结合施加牵引力来感知和响应基质。在这里,Arg - Gly - Asp (RGD)肽被共价偶联到双链DNA (dsDNA)和茎环DNA (slDNA)链上,具有43pN的张力耐量,并固定在PEG底物上。与dsDNA在高压下破裂导致RGD脱落不同,slDNA即使破裂也能保持结合。我们的研究结果表明,细胞通过调节肌动蛋白丝聚合和cofilin磷酸化来调节其粘附状态,有效地平衡talin构象以防止dsDNA断裂并维持正常粘附。这种现象被称为整合素-配体偶联强度,介导细胞适应性机械感应。此外,我们证明了正硬性可以转变为负硬性,这取决于整合素配体的偶联强度。这项研究强调了细胞-细胞外基质(ECM)相互作用中耦合强度的重要性,并为设计具有可调粘附性能的生物材料提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tunable Integrin–Ligand Coupling Strength Modulates Cellular Adaptive Mechanosensing
Cells sense and respond to the matrix by exerting traction force through binding of integrins to an integrin-specific ligand. Here, Arg−Gly−Asp (RGD) peptide is covalently conjugated to the double-stranded DNA (dsDNA) and stem-loop DNA (slDNA) tethers with a tension tolerance of 43pN and immobilized on a PEG substrate. Unlike dsDNA, which is ruptured under high tension, leading to the removal of RGD, slDNA remains bound even when ruptured. Our results suggest that cells adapt their adhesion state by modulating actin filament polymerization and cofilin phosphorylation, effectively balancing the talin conformation to prevent dsDNA rupture and maintain normal adhesion. This phenomenon, termed integrin–ligand coupling strength, mediated cellular adaptive mechanosensing. Furthermore, we demonstrate that positive durotaxis can shift to negative durotaxis, depending on the integrin–ligand coupling strength. This study highlights the significance of the coupling strength in cell–extracellular matrix (ECM) interactions and offers new insights into designing biomaterials with tunable adhesive properties for cell-based applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


