双咪唑盐离子液体催化剂催化碳酸二甲酯绿色合成异山梨酯基聚碳酸酯
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
针对可再生资源开发生物基聚合物的需求,提出了以二氧化碳基碳酸二甲酯(DMC)和生物基异山梨酯(ISB)为原料,离子液体(IL)催化熔融缩聚合成聚碳酸异山梨酯(PIC)的途径。设计了咪唑同时作为阴离子和正离子的1-乙基-3-甲基咪唑咪唑([Emim][Im]),与其他单咪唑正离子或阴离子的催化剂相比,其合成PIC的ISB转化率为98 %,PIC分子量为52600 g·mol−1,活性最高。[Emim][Im]的高催化活性可归因于其极高的阳离子静电势(+53.05 kcal/mol)和极低的阴离子静电势(- 76.12 kcal/mol),这两者共同导致了最强的相对亲电性和亲核性。此外,静电互补性的降低削弱了阳离子和阴离子之间的离子对相互作用,从而增强了催化过程中活性位点的可及性。这一过程将促进生物基聚合物的可持续生产。本文章由计算机程序翻译,如有差异,请以英文原文为准。

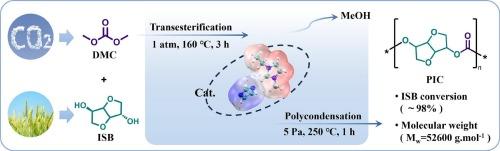
Green synthesis of isosorbide-based polycarbonate via dimethyl carbonate by dual imidazolate ionic liquid catalyst
Addressing the increased demand for the development of bio-based polymers from renewable resources, a pathway for synthesizing poly(isosorbide carbonate) (PIC) via melt polycondensation catalyzed by ionic liquid (IL) was proposed from CO2-based dimethyl carbonate (DMC) and bio-based isosorbide (ISB) as reactants. 1-Ethyl-3-methylimidazolium imidazolate ([Emim][Im]) with imidazole acted as both an anion and a cation was designed and the highest activity with an ISB conversion of 98 % and PIC molecular weight of 52600 g·mol−1 was achieved for the PIC synthesis compared to other catalysts with single imidazole cation or anion. High catalytic activity of [Emim][Im] can be attributed to its exceptionally high cationic electrostatic potential (+53.05 kcal/mol) and remarkably low anionic electrostatic potential (−76.12 kcal/mol), which collectively result in the strongest relative electrophilicity and nucleophilicity. Furthermore, the reduced electrostatic complementarity weakens the ion-pair interactions between the cation and anion, thereby enhancing the accessibility of active sites during the catalytic process. This process will promote the sustainable production of bio-based polymers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


