锰- na2wo4基催化剂上li2co3诱导甲烷氧化偶联中C2H4/C2H6选择性增强的基本原理
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
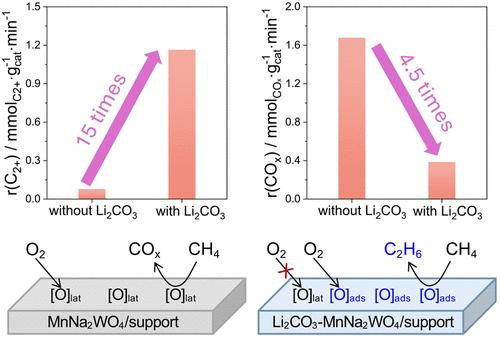
甲烷(OCM)与C2H6和C2H4 (c2 -碳氢化合物)的氧化偶联是一种工业上有吸引力的甲烷增值方法。然而,其对c2 -烃类,特别是对C2H4的选择性较低,阻碍了其商业化。在这项研究中,我们展示了使用基于Mn-Na2WO4体系的Li2CO3或LiNO3的物理混合物和不同负载的催化剂,以O2作为氧化剂有效地连续形成目标产物。当CH4转化率为7.2和13.3%时,Li2CO3-Mn-Na2WO4/Siral70 (AlSiOx的SiO2质量分数为70%)催化剂对c2 -烃类和C2H4的选择性分别为90.5和35.7%,87.6和43.3%,而不含li2co3的催化剂对C2H4和C2H4的选择性为无选择性。通过用Li2CO3促进支撑,然后沉积活性组分,可以获得非常相似的性能。原位温度分辨和非原位x射线衍射表征研究表明,促进剂与活性组分和载体完全反应,生成具有低比表面积的高结晶材料。这种结构调整抑制了甲烷直接非均相氧化生成碳氧化物,从而大大提高了对c2 -碳氢化合物的选择性。采用16O2或18O2的OCM进料进行稳态同位素瞬态动力学分析,并对产物进行时间分析,对选择性变化进行了合理化分析。li2co3诱导的相修饰影响了导致CO2和CO的表面中间体的浓度和寿命。所获得的知识为开发具有提高CO2选择性的OCM催化剂提供了指导。此外,获得的结果证明了我们的方法在阐明与定制催化剂设计相关的选择性控制因素方面的潜力,并可能刺激未来研究各种烷烃氧化反应中选择性催化剂的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fundamentals of Li2CO3-Induced Enhancement of C2H4/C2H6 Selectivity in Oxidative Coupling of Methane over Mn-Na2WO4-Based Catalysts
Oxidative coupling of methane (OCM) to C2H6 and C2H4 (C2-hydrocarbons) is an industrially attractive method for methane valorization. However, its commercialization is hampered by the low selectivity to C2-hydrocarbons, especially to C2H4. In this study, we demonstrate the use of a physical mixture of Li2CO3 or LiNO3 with differently supported catalysts based on the Mn-Na2WO4 system for the efficient continuous formation of the target products using O2 as an oxidant. The selectivity to C2-hydrocarbons and C2H4 of 90.5 and 35.7% or 87.6 and 43.3%, respectively, was obtained at 7.2 or 13.3% CH4 conversion over the Li2CO3-Mn-Na2WO4/Siral70 (AlSiOx with 70 wt % SiO2) catalyst, while its Li2CO3-free counterpart was unselective. Very similar performance is achieved by promoting the support with Li2CO3 followed by deposition of the active components. In situ temperature-resolved and ex situ X-ray diffraction characterization studies showed that the promoter reacted completely with the active components and the support to yield highly crystalline materials with a low specific surface area. Such restructuring resulted in a strong improvement in the selectivity to C2-hydrocarbons due to inhibiting the direct heterogeneous oxidation of methane to carbon oxides. The selectivity changes were rationalized by steady-state isotopic transient kinetic analysis using OCM feeds with 16O2 or 18O2 and temporal analysis of products. Li2CO3-induced phase modifications affect both the concentration and lifetime of surface intermediates leading to CO2 and CO. The knowledge gained provides guidance for the development of OCM catalysts with improved C2-selectivity. Moreover, the results obtained demonstrate the potential of our approach to elucidate the selectivity-governing factors relevant for tailored catalyst design and may stimulate future investigations of the development of selective catalysts in various alkane oxidation reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


