ALK在癌症中的作用:从功能到治疗靶向
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
间变性淋巴瘤激酶(ALK)是一种受体酪氨酸激酶(RTK),在儿童和成人的实体和血液系统恶性肿瘤中起致癌驱动作用。尽管ALK表达(ALK+)肿瘤对目前可用的一系列ALK抑制剂表现出强烈的初始反应,但许多患者会产生耐药性。在这篇综述中,我们讨论了ALK致癌信号传导的最新进展,以及现有的和有希望的治疗ALK驱动肿瘤的新方法,包括目前批准的ALK定向治疗,即酪氨酸激酶抑制剂,以及ALK特异性免疫治疗等新方法。虽然ALK抑制剂已经改变了ALK+肿瘤的管理和临床病史,但它们仍然不足以治愈大多数患者。因此,需要更多的努力来进一步改善结果,防止许多ALK+肿瘤患者经历的肿瘤耐药、复发和转移性扩散。在这里,我们概述了针对ALK和其他维持ALK+肿瘤持续性的基本途径的多管齐下的方法,以及有效或特异性免疫疗法如何实现这一目标。我们设想,从临床治疗ALK+肿瘤中吸取的经验教训,最终可以加速创新联合疗法的实施,以治疗由其他酪氨酸激酶或具有类似特性的癌基因驱动的肿瘤。本文章由计算机程序翻译,如有差异,请以英文原文为准。

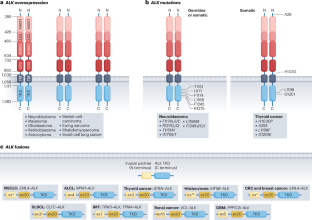
ALK in cancer: from function to therapeutic targeting
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase (RTK) that acts as an oncogenic driver in solid and haematological malignancies in both children and adults. Although ALK-expressing (ALK+) tumours show strong initial responses to the series of ALK inhibitors currently available, many patients will develop resistance. In this Review, we discuss recent advances in ALK oncogenic signalling, together with existing and promising new modalities to treat ALK-driven tumours, including currently approved ALK-directed therapies, namely tyrosine kinase inhibitors, and novel approaches such as ALK-specific immune therapies. Although ALK inhibitors have changed the management and clinical history of ALK+ tumours, they are still insufficient to cure most of the patients. Therefore, more effort is needed to further improve outcomes and prevent the tumour resistance, recurrence and metastatic spread that many patients with ALK+ tumours experience. Here, we outline how a multipronged approach directed against ALK and other essential pathways that sustain the persistence of ALK+ tumours, together with potent or specific immunotherapies, could achieve this goal. We envision that the lessons learned from treating ALK+ tumours in the clinic could ultimately accelerate the implementation of innovative combination therapies to treat tumours driven by other tyrosine kinases or oncogenes with similar properties. ALK genetic alterations drive various malignancies through aberrant tyrosine kinase activation, promoting oncogenic cell growth and survival. Here, Voena et al. outline recent advances in ALK oncogenic signalling, mechanisms of resistance, and both existing and promising new modalities to treat ALK-driven tumours.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


