IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在大型治疗药物通过血脑屏障(BBB)的受体介导转运(RMT)过程中,构建物(大分子或带有治疗载荷的较大载体)与脑毛细血管内皮细胞(BCEC)上的蛋白质结合,内化并释放到脑实质中。构建物在 BCEC 内的内化、跨 BCEC 的运输和释放,以及可能在 BCEC 内的滞留都受到其工程特性的影响,对其进行优化有助于深入了解 BCEC 的运输机制。此外,多组学的进步以及大规模筛选和定向进化活动也有助于确定 BCEC 的 RMT 新靶标。在这一视角中,我提出并思考了一些基本问题,这些问题可以通过比较 BBB 靶向构建物的工程特性和不同靶蛋白的特性而得出。这些问题涉及构建物工程特性的优化似乎趋于一致的潜在转运促进因素、靶蛋白在 RMT 中的确切作用、这些靶蛋白可能介导构建物转运的不同机制,以及在 BCEC 上选择靶蛋白的暂定标准。基于这些考虑,我提出了几种方案和策略来干扰构建物的运输,以便更有效地内化、通过内体网络向腔膜运输以及从 BCEC 释放,无论是对于较小的大分子还是较大的载体。本文章由计算机程序翻译,如有差异,请以英文原文为准。

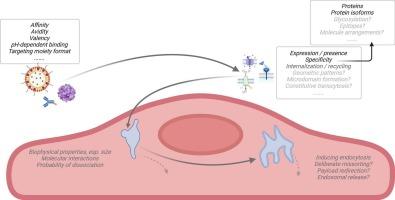
Mechanisms of receptor-mediated transcytosis at the blood-brain barrier
In receptor-mediated transcytosis (RMT) of large therapeutics across the blood-brain barrier (BBB), the construct - a macromolecule or a larger carrier with therapeutic payload - binds a protein on brain capillary endothelial cells (BCEC), with internalization and release into the brain parenchyma. The construct's internalization into, trafficking across and release from, but also possible entrapment within BCEC are affected by its engineered properties whose optimization has helped derive insights into transport mechanisms at BCEC. Furthermore, advances in multi-omics, as well as large-scale screening and directed evolution campaigns have helped identify new targets for RMT at BCEC.
In this perspective, I raise and reflect on some fundamental questions one can arrive at by comparing the engineered properties of BBB-targeted constructs and the properties of different target proteins. These questions concern the underlying, transcytosis-promoting factors that the optimization of constructs' engineered properties appears to converge on, the precise role of target proteins in RMT, the different mechanisms through which these targets may mediate construct trafficking, and the tentative criteria for target selection on BCEC. Based on these considerations I propose several scenarios and strategies to interfere with the construct's trafficking for more efficient internalization, transport through the endosomal network toward the abluminal membrane, and release from BCEC, both for smaller macromolecules and for larger carriers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


