合并对映选择性路易斯碱有机催化与金(I)催化:一锅法获得手性熔融多环化合物
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-21
DOI:10.1021/acs.joc.5c0005110.1021/acs.joc.5c00051
引用次数: 0
摘要
本文报道了一种在一锅顺序过程中利用两个不友好催化循环制备手性杂芳烃多环化合物的途径。α-杂芳烃-γ-丁内酯与炔功能化的森塔-贝里斯-希尔曼(MBH)碳酸盐进行了高度区域选择性、非对映选择性和对映选择性路易斯碱不对称烯丙基烷基化反应。值得庆幸的是,由于低路易斯碱催化剂负载,随后金催化的弗里德尔-克拉夫特型环化,包括形成融合的多环化合物,有效地进行,提供结构复杂,高度富集对映体的产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
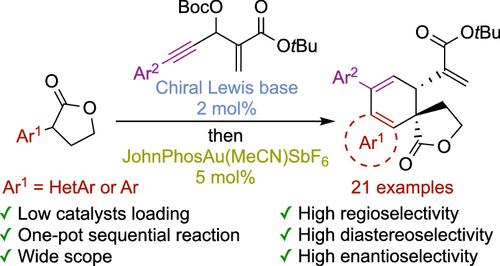
Merging Enantioselective Lewis Base Organocatalysis and Gold(I) Catalysis: A One-Pot Access to Chiral-Fused Polycyclic Compounds
Reported herein is a route to functionalized chiral heteroaromatic polycyclic compounds leveraging two unfriendly catalytic cycles in a one-pot sequential process. α-Heteroaromatic-γ-butyrolactones were engaged in a highly regio-, diastereo-, and enantio-selective Lewis base asymmetric allylic alkylation (AAA) with alkyne-functionalized Morita–Baylis–Hillman (MBH) carbonates. Gratefully, due to the low Lewis base catalyst loading, subsequent gold-catalyzed Friedel–Crafts type cyclization, entailing the formation of fused polycyclic compounds, proceeded efficiently, affording structurally complex, highly enantioenriched products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


