Cu(II)催化氨基接种对吲哚的c3 -异芳基化反应及邻烷基苯胺的亲核加成反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-23
DOI:10.1021/acs.joc.5c0000710.1021/acs.joc.5c00007
引用次数: 0
摘要
过渡金属催化邻炔基苯胺的氨基金属化反应是合成c3功能化吲哚的一个很有前途的途径。在此,我们描述了Cu(II)催化的邻烷基苯胺和喹啉n -氧化物的吲哚的位点选择性c3 -杂芳化反应。提出了一种可能的反应机理,即邻炔基苯胺氨基接种,然后在喹啉n -氧化物上亲核加成Cu(II)-吲哚配合物。生成的c3 -杂芳化吲哚的后转化表明了所开发方法的广泛适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
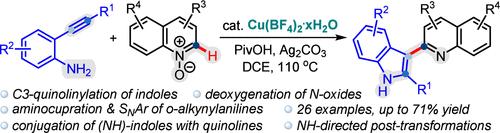
C3-Heteroarylation of Indoles via Cu(II)-Catalyzed Aminocupration and Subsequent Nucleophilic Addition of o-Alkynylanilines
Transition-metal-catalyzed aminometalation of o-alkynylanilines is a promising pathway for the synthesis of C3-functionalized indoles. Herein, we describe the Cu(II)-catalyzed site-selective C3-heteroarylation of indoles from o-alkynylanilines and quinoline N-oxides. A plausible reaction mechanism involving the aminocupration of o-alkynylanilines followed by the nucleophilic addition of Cu(II)-indolyl complexes to quinoline N-oxides was proposed. Post-transformations of the generated C3-heteroarylated indoles demonstrated the broad applicability of the developed method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


