配体主链半游离臂和酰胺官能团对单核铜(II)配合物催化化学和电化学双氧还原的影响
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
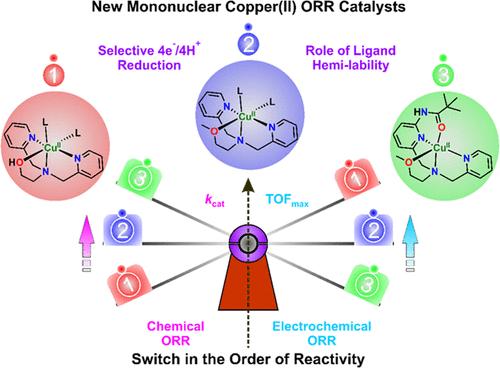
本文分离了四种柔性配体的单核铜(II)配合物,[(DPA- oh)Cu(CH3OH)(ClO4)](ClO4)(1)、[(DPA- ome)Cu(CH3OH)(ClO4)](ClO4)(2)、[(6-酰胺-DPA- ome)Cu](ClO4)2(3)和[(6-酰胺-DPA- ome)Cu](ClO4)2(4),这些配体在DPA(二(2-吡啶)胺)骨架上具有半可降解(羟基)甲氧乙基和/或酰胺基,以探索它们催化化学和电化学氧还原反应(ORR)的能力。研究了半活性臂和酰胺在配体主链上的功能对整个催化循环的速率决定步骤(RDS)的影响,并与类似的[(tmpa)Cu](ClO4)2 (tmpa =三(2-吡啶基甲基)胺)和[(PV-tmpa)Cu](ClO4)2 (PV-tmpa =双(吡啶-2-基甲基){[6-(pivalamido)吡啶-2-基甲基]-胺)配合物进行了比较。在这些配合物的半可降解臂导致整体的三阶速率,在双氧还原催化过程中kcat值在103到104 M-2s-1之间。除配合物4外,其余配合物均以十甲基二茂铁(Fc*)为牺牲还原剂,在298 K的酸性丙酮中选择性地通过4e - /4H+还原途径还原为水(H2O)。相反,在磷酸盐缓冲液中,将反应条件从化学环境改变为电化学环境,在保持与丙酮化学ORR催化相同的产物选择性的同时,配合物的ORR活性呈现相反的顺序。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of Hemilabile Arm and Amide Functionality in the Ligand Backbone on Chemical and Electrochemical Dioxygen Reduction Catalyzed by Mononuclear Copper(II) Complexes
Four mononuclear copper(II) complexes, [(DPA-OH)Cu(CH3OH)(ClO4)](ClO4) (1), [(DPA-OMe)Cu(CH3OH)(ClO4)](ClO4) (2), [(6-Amide-DPA-OMe)Cu](ClO4)2 (3) and [(6-Amide2-DPA-OMe)Cu](ClO4)2 (4), of flexidentate ligands bearing hemilabile (hydroxy)methoxyethyl and/or amide group on DPA (di(2-picolyl)amine) backbone were isolated to explore their potency in catalyzing chemical and electrochemical oxygen reduction reaction (ORR). The role of a hemilabile arm, as well as amide functionality on the ligand backbone in affecting the rate-determining step (RDS) of the overall catalytic cycle has been explored and compared with that of the analogous [(tmpa)Cu](ClO4)2 (tmpa = tris(2-pyridylmethyl)amine) and [(PV-tmpa)Cu](ClO4)2 (PV-tmpa = bis(pyrid-2-ylmethyl){[6-(pivalamido)pyrid-2-yl]methyl}-amine) complexes. The hemilabile arm in these complexes results in an overall third-order rate, with kcat values ranging from 103 to 104 M–2s–1 during dioxygen reduction catalysis. All the complexes except complex 4 selectively reduce dioxygen via the 4e–/4H+ reduction pathway to water (H2O) using decamethylferrocene (Fc*) as a sacrificial reductant in acidic acetone at 298 K. In contrast, altering the reaction conditions from a chemical to an electrochemical ambiance in phosphate buffer displays a reverse order of ORR activity of the complexes while maintaining the same product selectivity as observed in chemical ORR catalysis in acetone.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


