通过肠-肝轴编辑肠道微生物群以减轻结肠炎和维持肝脏稳态的益生菌递送
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
炎症性肠病(IBD)破坏肠道屏障,破坏肠道微生物群,通过肠-肝轴影响肝功能,进而通过脂质代谢物影响肠道微生物群,加剧IBD。本研究介绍了一种以益生菌为基础的治疗方法,将嗜酸乳杆菌包裹在负载钨离子的介孔聚多巴胺(LA@WMPDA)中,以改善结肠炎和平衡肠肝内稳态。经口服后,该胶囊可保护嗜酸乳杆菌,清除活性氧/氮,释放的钨离子可抑制结肠炎期间肠杆菌科的异常生长,从而恢复肠道屏障,调节肠道菌群。非靶向代谢组学和转录组学分析显示短链脂肪酸和吲哚衍生物增加,肝脏脂质代谢降低。在结肠和肝脏转录组分析中,与免疫反应、细胞迁移和死亡以及对细菌的反应相关的途径显示出显著的下调。因此,该研究为IBD治疗提供了一个开创性的范例,并强调了通过肠-肝轴调节肝脏相关代谢功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
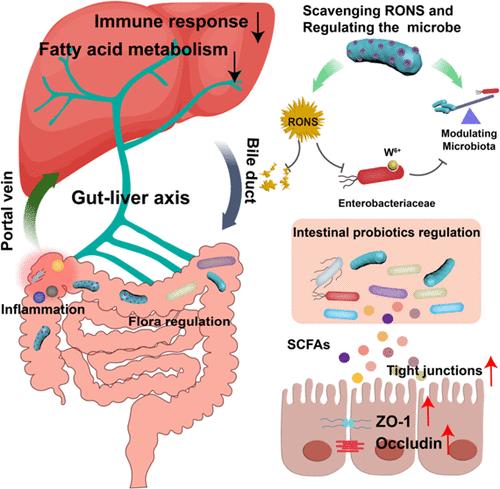
Probiotic Delivery for Editing of the Gut Microbiota to Mitigate Colitis and Maintain Hepatic Homeostasis Via Gut–Liver Axis
Inflammatory bowel disease (IBD) compromises the intestinal barrier and disrupts gut microbiota, impacting liver function via the gut–liver axis, which in turn influences the intestinal microbiota through lipid metabolites exacerbating IBD. This study introduced a probiotic-based treatment using Lactobacillus acidophilus encapsulated in tungsten ion-loaded mesoporous polydopamine (LA@WMPDA) to ameliorate colitis and balance enterohepatic homeostasis. After oral administration, the encapsulation could protect Lactobacillus acidophilus, scavenge reactive oxygen/nitrogen species, and the released tungsten ions would inhibit abnormal Enterobacteriaceae growth during colitis, consequently restoring the intestinal barrier and regulating the gut microbiota. Nontargeted metabolomics and transcriptomics analyses showed increased short-chain fatty acids and indole derivatives, and decreased hepatic lipid metabolism. Pathways associated with immune response, cell migration and death, and response to bacterium showed significant down-regulation in the colon and liver transcriptome analysis. Thus, this study provided a pioneered paradigm for IBD treatment and highlighted the regulation of liver-related metabolic functions via the gut–liver axis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


