抑制高倍率钠离子电池中Na3MnTi(PO4)3电压滞后的配位化学调控
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
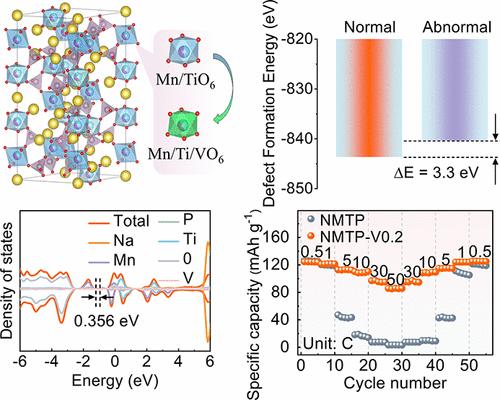
nasiconon型Na3MnTi(PO4)3 (NMTP)作为一种钠离子超离子导体,由于其高稳定性和高功率密度,在大型钠离子电池中越来越受到关注。然而,由于内在动力学迟缓和严重的结构降解,它的倍率能力较差,循环寿命较短。本文采用钒(V)作为掺杂剂,在NMTP中等量取代锰(Mn)和钛(Ti),以减轻电压滞后,提高循环性能。v掺杂调节了过渡金属的局部配位化学,降低了循环过程中衍生的反位缺陷浓度。通过密度泛函理论分析,Na3Mn0.9V0.2Ti0.9(PO4)3 (NMTP-V0.2)具有较低的带隙和较高的电导率。此外,v掺杂显著降低了Na2的扩散势垒,导致在Mn2+/Mn3+氧化还原过程中,Na+的扩散率比NMTP高出约两个数量级。制备的NMTP-V0.2在50℃下具有85.3 mAh g-1的优异倍率,并且在1400次循环中具有81%的令人满意的循环保持率。因此,组装的NMTP-V0.2/硬碳钠离子全电池在10℃下循环500次后,能量密度高达292.3 Wh kg-1,容量保持率高达92%。这一结果不仅为抑制多阴离子阴极的电压滞后提供了途径,也为高功率sib的设计提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Coordination Chemistry Regulation Suppressing Voltage Hysteresis for Na3MnTi(PO4)3 in High-Rate Sodium-Ion Batteries
As a natrium superionic conductor, NASICON-type Na3MnTi(PO4)3 (NMTP) has garnered increasing attention for large-scale sodium-ion batteries due to its high stability and power densities. Nevertheless, it still suffers from an inferior rate capability and poor cycling longevity, arising from sluggish intrinsic kinetics and severe structural degradation. Herein, vanadium (V) is used as a dopant for equal substitution of manganese (Mn) and titanium (Ti) in NMTP to alleviate voltage hysteresis and enhance the cycling performance. V-doping regulates the local coordination chemistry of transition metals and reduces derivative antisite defect concentration upon cycling. Through density functional theory analysis, Na3Mn0.9V0.2Ti0.9(PO4)3 (NMTP-V0.2) demonstrates a lower bandgap and higher electronic conductivity. Additionally, V-doping significantly lowers the diffusion barrier of Na2, leading to Na+ diffusivity that is approximately two orders of magnitude higher than that of NMTP during the Mn2+/Mn3+ redox process. The as-prepared NMTP-V0.2 delivers an excellent rate capability of 85.3 mAh g–1 under 50 C and satisfactory cycling retention of 81% with a high capacity over 1400 cycles. Thus, the assembled NMTP-V0.2/hard carbon sodium-ion full cell achieves a high energy density of 292.3 Wh kg–1 as well as outstanding capacity retention of 92% after 500 cycles under 10 C. This result not only provides an approach for suppressing voltage hysteresis in polyanion cathodes but also offers guidance for designing high-power SIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


