一种新的al18f标记的nota修饰泛素29-41衍生物作为细菌感染PET显像剂
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
抗菌肽泛素29-41 (TGRAKRRMQYNRR)是检测细菌感染的潜在靶点。合成了一种以赖氨酸氨基侧1,4,7-三氮杂环壬烷-1,4,7-三乙酸(NOTA)修饰的新型UBI 29-41衍生物,并用Al18F进行放射性标记,命名为[18F]AlF-NOTA-UBI 29-41。新型PET示踪剂在室温生理盐水和37℃小鼠血清中均保持良好的体外稳定性。体外细菌结合实验表明,该示踪剂能特异性结合金黄色葡萄球菌。感染肌肉和发炎肌肉对[18F]AlF-NOTA-UBI 29-41的摄取在生物分布上有显著差异。细菌感染小鼠模型的PET成像研究显示,在感染部位可见积聚,表明该复合物是区分细菌感染和无菌炎症的潜在PET示踪剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
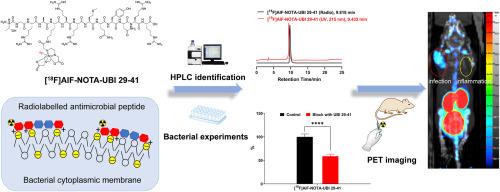
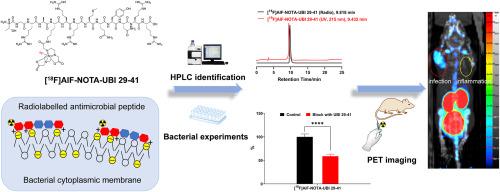
A novel Al18F-labelled NOTA-modified ubiquicidin 29-41 derivative as a bacterial infection PET imaging agent
The antimicrobial peptide ubiquicidin 29-41 (TGRAKRRMQYNRR) is a potential target for detecting bacterial infection. A novel UBI 29–41 derivative modified with 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) on the amino side of lysine was synthesized and radiolabelled with Al18F, named [18F]AlF-NOTA-UBI 29–41. The novel PET tracer maintained good in vitro stability in saline at room temperature and mouse serum at 37 °C. In vitro bacterial binding experiments indicated that the tracer specifically bound to Staphylococcus aureus. A significant difference in the uptake of [18F]AlF-NOTA-UBI 29–41 between infected muscle and inflamed muscle was observed in biodistribution. A PET imaging study in mouse models with bacterial infection and sterile inflammation showed apparent accumulation at the infection site, suggesting that the complex is a potential PET tracer for distinguishing bacterial infection from sterile inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


