从多方面优化 CO2/H2O 活化对食物垃圾生物炭表面特性的影响:去除水中铅(II)的高效绿色吸附剂
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
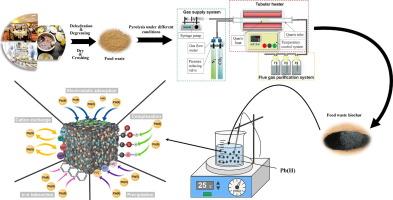
Multifaceted optimization of CO2/H2O activation on the surface properties of food waste biochar: An efficient green adsorbent for the removal of Pb(II) from water
In this study, an adsorbent (CHBC) with good adsorption performance for Pb(II) was successfully prepared by CO2/H2O activation of food waste biochar. Specifically, the specific surface area of CHBC reached 144 m2·g−1, which was 48 times higher than that of unactivated food waste biochar, and the adsorption of Pb(II) by CHBC was a spontaneous heat-absorption reaction, with a maximum Pb(II) adsorption capacity of 114.59 mg·g−1, which can be well described by the pseudo-second-order kinetic model and the Freundlich isothermal adsorption model. The adsorption process of Pb(II) on CHBC is dominated by chemisorption, and the adsorption mechanism includes the ion-exchange reaction of Pb(II) with Ca2+ on CHBC, the precipitation reactions with phosphate and SO32-, the complexation reactions with oxygen-containing functional groups such as –OH and C=O, the electrostatic attraction, and the Pb(II)-π interactions. The combination of these mechanisms results in an excellent adsorption capacity for Pb(II) for CHBC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


