胶体金属氧化物纳米颗粒的氧原子转移反应
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属氧化物(MOx)/溶液界面上的氧化还原转化非常重要,氧原子转移(OAT)是这种反应性的最简单和最基本的例子之一。OAT是一种双电子转移过程,在气固反应和催化中很有名。然而,OAT很少在氧化物/水界面直接观察到,其氧化还原反应通常被认为发生在单电子步骤中。本文报道了有机分子与水性胶体二氧化钛和氧化铱纳米粒子(TiO2和IrOx NPs)的化学计量OAT反应。Me2SO (DMSO)氧化还原的TiO2 NPs生成Me2S, IrOx NPs将O原子转化为水溶性膦和硫醚。利用胶体的高比表面积和高透明度,建立了反应化学计量学,并利用典型的溶液光谱技术探讨了其化学机理。这些OAT反应,包括催化反应,利用单个NPs积累许多电子和/或空穴的能力。观察两种不同材料在相反方向上的OAT反应,是利用氧化物纳米颗粒进行有价值的多电子和多空穴转化的一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
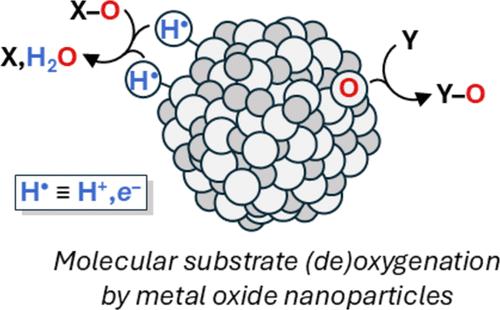
Oxygen Atom Transfer Reactions of Colloidal Metal Oxide Nanoparticles
Redox transformations at metal oxide (MOx)/solution interfaces are broadly important, and oxygen atom transfer (OAT) is one of the simplest and most fundamental examples of such reactivity. OAT is a two-electron transfer process, well-known in gas/solid reactions and catalysis. However, OAT is rarely directly observed at oxide/water interfaces, whose redox reactions are typically proposed to occur in one-electron steps. Reported here are stoichiometric OAT reactions of organic molecules with aqueous colloidal titanium dioxide and iridium oxide nanoparticles (TiO2 and IrOx NPs). Me2SO (DMSO) oxidizes reduced TiO2 NPs with the formation of Me2S, and IrOx NPs transfer O atoms to a water-soluble phosphine and a thioether. The reaction stoichiometries were established and the chemical mechanisms were probed using typical solution spectroscopic techniques, exploiting the high surface areas and transparency of the colloids. These OAT reactions, including a catalytic example, utilize the ability of the individual NPs to accumulate many electrons and/or holes. Observing OAT reactions of two different materials, in opposite directions, is a step toward harnessing oxide nanoparticles for valuable multi-electron and multi-hole transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


