硫酸锌电解液中超低浓度尿素衍生物对锌离子电池超长循环寿命的综合实验研究
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在本研究中,一种低成本、友好的化合物二唑烷脲(diazolidinylurea)作为尿素(DU)的衍生物,携带大量杂原子和氢键,在ZnSO4电解质中有效地强化了水性锌离子电池(AZIBs)。采用多种表面化学和电化学手段,研究了DU对2 M硫酸锌电解液中锌阳极的影响。结果表明,超低浓度的DU (2 mM, 0.00556 wt %)可以抑制水系zib恒流循环过程中锌枝晶的形成、锌的腐蚀和析氢反应,从而使对称锌锌电池在1 mA·cm-2、1 mA h·cm-2条件下的循环寿命达到7336 h(近306天),在25℃条件下的循环寿命达到426 h(近18天)。激发锌锰全电池在2 a·g-1电流下循环1000次后,容量保留率保持在52%以上。这些结果远远优于基于空白硫酸锌电池的锌离子电池。即使含有DU/ 2m ZnSO4电解质的Zn-Cu半电池也比未含ZnSO4电解质的半电池更明显。在硫酸锌溶液中,DU对锌电极的最大缓蚀效率超过82%。因此,在硫酸锌电解质中添加DU抑制锌腐蚀和寄生副反应,以及锌枝晶的形成和生长,在强化锌离子电池中发挥了核心作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
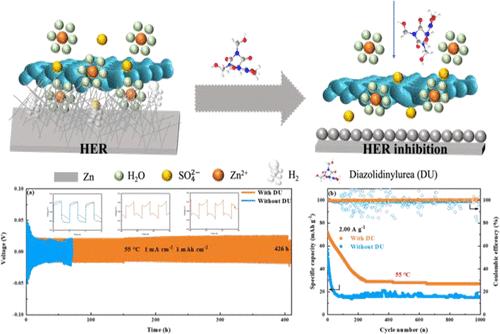
Comprehensive Experimental Insights into Ultra-Long Cycle Life of Zinc-Ion Batteries Inspired by Super Low Concentrations of a Derivative of Urea in the Zinc Sulfate Electrolyte
In this study, a low-cost and friendly compound diazolidinylurea carrying a number of heteroatoms and inter/intra hydrogen bonding, a derivative of urea (DU), efficiently strengthened aqueous zinc ions batteries (AZIBs) in ZnSO4 electrolyte. The influence of DU on the zinc anode in the 2 M ZnSO4 electrolyte was fully studied by various surface chemistry and electrochemistry means. It is demonstrated that the super low concentration of DU (2 mM, 0.00556 wt %) could inhibit the formation of zinc dendrites, zinc corrosion, and the hydrogen evolution reaction during the constant current cycling of water system ZIBs, which thus enabled symmetric zinc–zinc batteries to reach a cycle life of 7336 h (nearly 306 days) under 1 mA·cm–2, 1 mA h·cm–2 at 25 °C and 426 h (nearly 18 days) at 55 °C, and inspired zinc–manganese full battery to maintain a capacity retention rate of more than 52% after cycling for 1000 cycles under a current of 2 A·g–1. These results are much superior over zinc ion cells based on the blank ZnSO4 batteries. Even if the Zn–Cu half cells including the DU/2 M ZnSO4 electrolyte were also more pronounced than those with the bare ZnSO4 electrolyte. The maximum corrosion inhibition efficiency of the DU for the zinc electrode in ZnSO4 solution exceeded 82%. Hence, the suppression of zinc corrosion and parasitic side reactions, as well as the formation and growth of zinc dendritic crystals by the addition of DU in zinc sulfate electrolyte, played a central role in intensifying aqueous zinc ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


