利用多孔镍催化剂在膜电极组合电解槽中有效地将一氧化氮转化为氨
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氨的电化学合成为世界第二大商品化学品的脱碳和电气化生产提供了一条有前途的途径。在潜在的反应物中,NOx气体因其良好的热力学,有利的动力学以及燃烧排放和固氮过程(如等离子体诱导的大气氮氧化)的可用性而脱颖而出。然而,这些来源中典型的低浓度NOx对电化学性能构成了重大挑战,特别是由于反应物质量传输的限制。在这项工作中,我们报告了在膜电极组件(MEA)电解槽中使用多孔镍催化剂以直接使用稀释的一氧化氮(NO)进料。合理优化反应质量输运后,no - nh3单次转化率最高可达93%,氨的法拉第效率最高可达92%,铵的产率最高可达556 μmol/h⋅cm2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
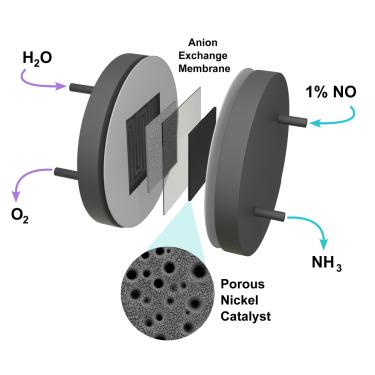
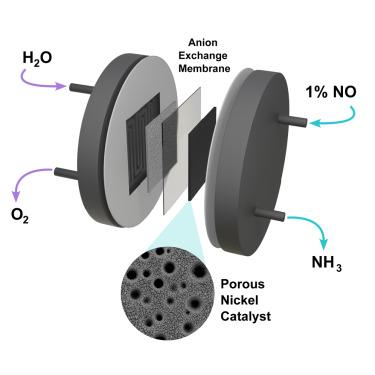
Efficient electrochemical conversion of nitric oxide to ammonia using a porous nickel catalyst in a membrane electrode assembly electrolyzer
The electrochemical synthesis of ammonia presents a promising pathway to decarbonize and electrify the production of the world’s second-largest commodity chemical. Among potential reactants, NOx gases stand out owing to their favorable thermodynamics, advantageous kinetics, and availability from both combustion emissions and nitrogen-fixation processes, such as plasma-induced atmospheric nitrogen oxidation. However, the typically low concentration of NOx in these sources poses significant challenges for electrochemical performance, particularly due to limitations in reactant mass transport. In this work, we report on the use of a porous nickel catalyst in a membrane electrode assembly (MEA) electrolyzer to enable the direct use of a dilute nitric oxide (NO) feed. The rational optimization of reactant mass transport led to the attainment of maximum values of NO-to-NH3 single-pass conversion of 93%, faradaic efficiency for ammonia of 92%, and ammonium production rate of 556 μmol/h⋅cm2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


