结合自旋涂覆和开环复分解聚合的半氟化共聚物膜减少氟碳
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
全氟和多氟烷基物质(PFAS)在社会中普遍存在,主要是由于其独特的表面性质,但与这些物质相关的重大健康问题强调了减少PFAS战略的必要性。我们报道了一种结合自旋涂覆和开环复分解聚合(scROMP)的方法,通过降冰片烯(NB)与5-(全氟-正烷基)降冰片烯(NBFn)的共聚,大幅度减少了PFAS的用量、溶剂和合成低表面能聚合物膜所需的时间。独特的scROMP方法有效地将聚合物薄膜的合成和沉积集成到一个快速的过程中,用1ml溶剂在2分钟内将单体转化为聚合物薄膜,形成36平方厘米的薄膜。研究了全氟烷基链长n为4,6和8的情况,氟碳成分倾向于支配所有n的表面,即使接触单体中只有2%的NBFn,其水接触角也与氟碳均聚物相当。作为一种潜在的应用,这些半氟化共聚物薄膜被用于乙醇脱水中,作为非晶含氟聚合物膜的低PFAS替代品。即使聚合物中氟碳含量为7%(或单体中氟碳含量为2%),与全碳氢化合物膜相比,选择性也会增加一个数量级,氟化程度最高可达63%(单体为50%),导致另一个数量级的增强,性能与pNBFn均聚物相似。此外,致密的外层氟碳层为估计膜内氟碳和碳氢化合物基团的选择性吸附和扩散组分提供了理想的设置。本文章由计算机程序翻译,如有差异,请以英文原文为准。
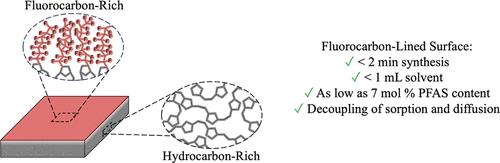
Fluorocarbon Minimization Via Semifluorinated Copolymer Films by Combining Spin Coating and Ring-Opening Metathesis Polymerization
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous in society largely due to their unique surface properties, but significant health concerns associated with these substances underscore the need for PFAS reduction strategies. We report a method to substantially reduce the amount of PFAS, solvent, and time needed to synthesize a low surface energy polymer film through the copolymerization of norbornene (NB) with 5-(perfluoro-n-alkyl)norbornenes (NBFn) in a single process that combines spin coating with ring-opening metathesis polymerization (scROMP). The unique scROMP approach efficiently integrates polymer film synthesis and deposition into one rapid process, converting monomer into polymer films in <2 min with <1 mL of solvent for a 36 cm2 film. Perfluoroalkyl chain lengths, n, of 4, 6, and 8 were examined, with the fluorocarbon component tending to dominate the surface for all n, exhibiting water contact angles comparable to those of the fluorocarbon homopolymer even with as little as 2% NBFn in the contacting monomer. As a potential application, these semifluorinated copolymer films were used in ethanol dehydration as low PFAS substitutes for amorphous fluoropolymer membranes. Even 7% fluorocarbon in the polymer (or 2% in the monomer) caused an order-of-magnitude increase in selectivity over a fully hydrocarbon membrane, with additional fluorination up to 63% (50% in monomer), leading to another order-of-magnitude enhancement and properties similar to the pNBFn homopolymer. Additionally, the dense outer fluorocarbon layer provided an ideal setup to estimate the sorption and diffusion components of selectivity for fluorocarbon and hydrocarbon groups within a membrane.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


