植物布尼亚病毒复制机制激活及利巴韦林双靶向抑制的结构基础
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
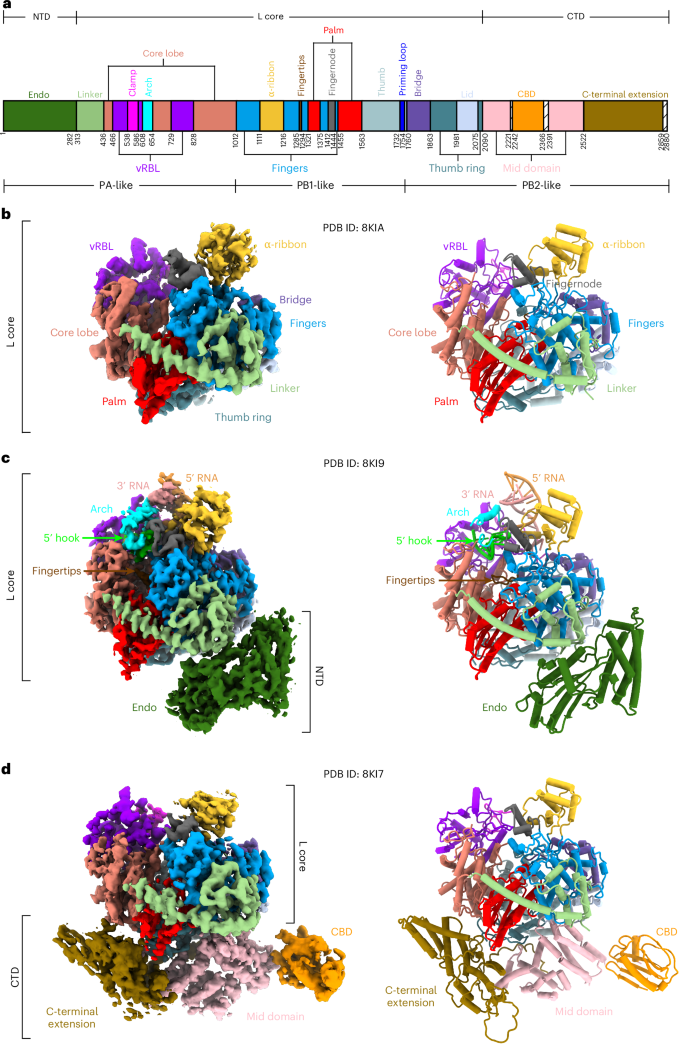
尽管植物病毒作为一类新的病原体早在一个多世纪前就已被发现,但植物病毒复制机器和抗病毒杀虫剂的结构仍然缺乏研究。在这里,我们报告了一种毁灭性植物布尼亚病毒--番茄斑点萎蔫病正交病毒(TSWV)中约 330 kDa 的 RNA 依赖性 RNA 聚合酶(RdRp)的五种低温电子显微镜结构,包括正常状态、病毒-RNA 结合状态、碱基类似物利巴韦林结合状态和利巴韦林-三磷酸结合状态。他们发现,RdRp motif F 的一个柔性环可作为 "传感器 "感知病毒 RNA,并进一步作为 "适配器 "促进完整催化中心的形成。十个碱基的 RNA "钩子 "结构足以引发主要构象变化并激活 RdRp。化学筛选显示,利巴韦林对 TSWV 有效,结构数据显示,利巴韦林破坏了钩状结合和催化核心的形成,使聚合酶锁定在非活性状态。这项工作从结构上揭示了植物布尼亚病毒 RdRp 激活及其双靶点抑制的机制,有助于开发针对植物病毒的杀虫剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Structural basis for the activation of plant bunyavirus replication machinery and its dual-targeted inhibition by ribavirin
Despite the discovery of plant viruses as a new class of pathogens over a century ago, the structure of plant virus replication machinery and antiviral pesticide remains lacking. Here we report five cryogenic electron microscopy structures of a ~330-kDa RNA-dependent RNA polymerase (RdRp) from a devastating plant bunyavirus, tomato spotted wilt orthotospovirus (TSWV), including the apo, viral-RNA-bound, base analogue ribavirin-bound and ribavirin-triphosphate-bound states. They reveal that a flexible loop of RdRp’s motif F functions as ‘sensor’ to perceive viral RNA and further acts as an ‘adaptor’ to promote the formation of a complete catalytic centre. A ten-base RNA ‘hook’ structure is sufficient to trigger major conformational changes and activate RdRp. Chemical screening showed that ribavirin is effective against TSWV, and structural data revealed that ribavirin disrupts both hook-binding and catalytic core formation, locking polymerase in its inactive state. This work provides structural insights into the mechanisms of plant bunyavirus RdRp activation and its dual-targeted site inhibition, facilitating the development of pesticides against plant viruses. There are more than 2,100 known plant virus species. This work presents full-length three-dimensional structures of a plant virus’s replication machinery, providing insights into its activation and inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


