胞苷mRNA被NAT10乙酰化在T细胞扩增和抗病毒免疫中的关键作用
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
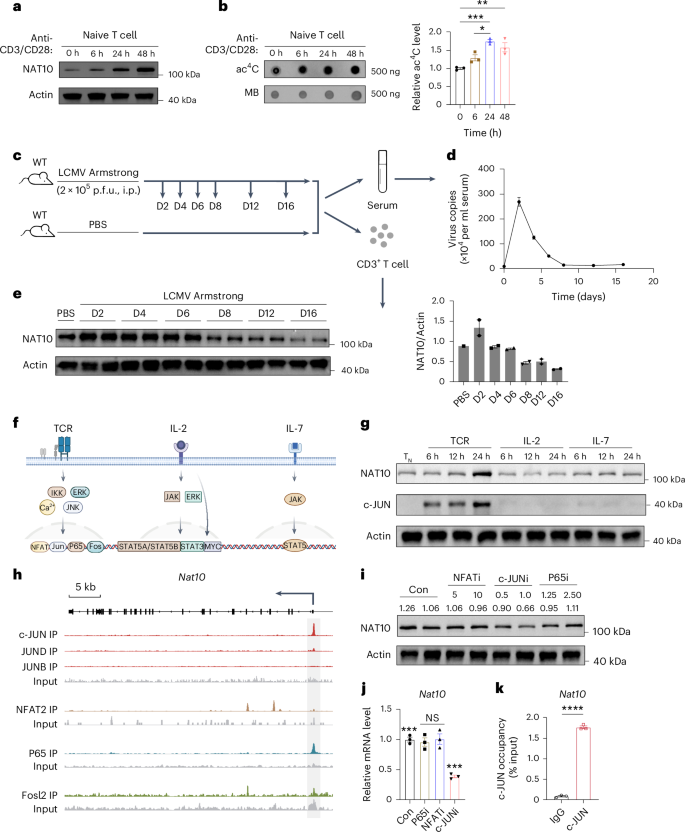
激活后,初始T细胞退出静止状态,需要全局翻译以快速扩增,但潜在的机制尚不清楚。在这里,我们发现在T细胞活化过程中,细胞上调n -乙酰基转移酶10 (NAT10)的表达,这是一种负责mrna的n4 -乙酰胞苷(ac4C)修饰的酶。ac4c修饰的Myc mrna具有更高的翻译效率,能够快速合成Myc蛋白并支持稳健的T细胞扩增。在急性淋巴细胞性脉络丛脑膜炎病毒模型中,小鼠T细胞中Nat10的条件缺失导致严重的细胞周期阻滞和细胞扩增限制,最终加剧感染。此外,NAT10水平较低的老年人的T细胞表现出增殖缺陷,这可能部分解释了老年人抗病毒反应受损的原因。该研究揭示了T细胞扩增、信号依赖性mRNA降解诱导的机制,以及ac4C修饰在T细胞介导的免疫应答中的潜在体内生物学意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。

A critical role of N4-acetylation of cytidine in mRNA by NAT10 in T cell expansion and antiviral immunity
Following activation, naive T cells exit quiescence and require global translation for rapid expansion, yet the underlying mechanisms remain unclear. Here, we show that during T cell activation, cells upregulate the expression of N-acetyltransferase 10 (NAT10), an enzyme responsible for N4-acetylcytidine (ac4C) modification of mRNAs. ac4C-modified Myc mRNAs show higher translation efficiency, enabling rapid synthesis of MYC protein and supporting robust T cell expansion. Conditional deletion of Nat10 in mouse T cells causes severe cell cycle arrest and limitation of cell expansion due to MYC deficiency, ultimately exacerbating infection in an acute lymphocytic choriomeningitis virus model. Additionally, T cells from older individuals with lower NAT10 levels show proliferative defects, which may partially account for impaired antiviral responses in older individuals. This study reveals a mechanism governing T cell expansion, signal-dependent mRNA degradation induction and the potential in vivo biological significance of ac4C modification in T cell-mediated immune responses. The authors show that NAT10 is upregulated with T cell antigen receptor signaling and that T cell-specific NAT10 deficiency results in diminished numbers of naive T cells, translating to an inability to mount a significant antiviral T cell response.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


