金/银催化n -烯丙基-2-炔基苯胺和α-重氮化合物通过1,3-烯丙基迁移合成功能化吲哚
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过α-重氮化合物将碳插入n -烯丙基-2-(芳基/烷基乙基)苯胺的N-H键,然后环化并伴随烯丙基片段的1,3迁移,金催化合成了修饰吲哚。开发的方案解决了直接C3功能化的遗传挑战,并且消除了对1,3迁移反应的叔苯胺前体的需要。这种转化的适用性通过实际合成类似药物的小分子和药学上相关的分子,如布洛芬、雌二醇、薄荷醇和冰片等的类似物来展示。控制实验和反应中间体的分离得到了很好的支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
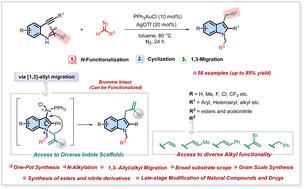
Gold/silver-catalyzed synthesis of functionalized indoles from N-allyl-2-alkynylanilines and α-diazo compounds via 1,3-allyl migration†
Gold-catalyzed synthesis of decorated indoles has been developed through carbene insertion into N–H bonds of N-allyl-2-(aryl/alkyl ethynyl)anilines using α-diazo compounds followed by cyclization and concomitant 1,3 migration of allyl fragments. The developed protocol tackles the inherited challenge of direct C3 functionalization and eliminates the need for a tertiary aniline precursor for the 1,3-migration reaction. The applicability of this transformation is showcased through the practical synthesis of analogs of small drug-like and pharmaceutically relevant molecules such as ibuprofen, estradiol, menthol, and borneol, etc. The mechanism is well supported by control experiments and isolation of the reaction intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


