基于药物团结构简化策略的新型载脂蛋白ido1抑制剂的发现和优化
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
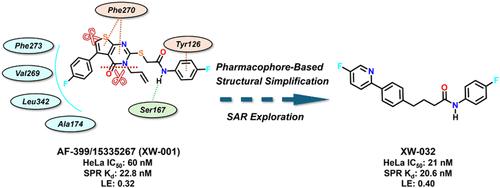
吲哚胺2,3-双加氧酶-1 (IDO1)在肿瘤免疫逃逸中起重要作用。然而,传统的IDO1抑制剂临床疗效有限,阻碍了其进一步发展。最近,apo-IDO1抑制剂取代血红素靶向IDO1已被发现,其解离速度慢,令人想起不可逆抑制剂。这一特点表明持续的目标接触,提供药效学优势。因此,开发载脂蛋白ido1抑制剂是ido1相关研究领域的一个有前景的策略。在这里,我们通过基于结构的虚拟筛选来鉴定噻吩嘧啶衍生物XW-001,然后通过迭代优化过程来开发XW-032。通过药物团引导的结构简化方法,XW-032对载脂蛋白ido1表现出显著的体外抑制活性(IC50 = 21±5 nM)。值得注意的是,XW-032 (TGI = 63%)在CT26同基因小鼠模型中表现出强大的体内抗肿瘤功效,突出了载脂蛋白ido1抑制剂在肿瘤免疫治疗中的益处。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and Optimization of Novel Apo-IDO1 Inhibitors by a Pharmacophore-Based Structural Simplification Strategy
Indoleamine 2,3-dioxygenase-1 (IDO1) plays a crucial role in tumor immune escape. However, the limited clinical efficacy of traditional IDO1 inhibitors has impeded their further development. Recently, apo-IDO1 inhibitors that displace the heme to target IDO1 have been discovered, which exhibits a slow dissociation rate reminiscent of irreversible inhibitors. This characteristic suggests sustained target engagement, offering a pharmacodynamic advantage. Therefore, the development of apo-IDO1 inhibitors emerges as a promising strategy in the field of IDO1-related studies. Here, we present the identification of the thienopyrimidine derivative XW-001 through structure-based virtual screening, followed by an iterative optimization process that led to the development of XW-032. XW-032 exhibited remarkable in vitro inhibitory activity against apo-IDO1 (IC50 = 21 ± 5 nM) through a pharmacophore-guided structural simplification approach. Notably, XW-032 (TGI = 63%) exhibited potent in vivo antitumor efficacy in the CT26 syngeneic mouse model, highlighting the benefits of apo-IDO1 inhibitors for tumor immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


