异聚体烟碱乙酰胆碱受体上正位配体结合特性的非均相动力学起源
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
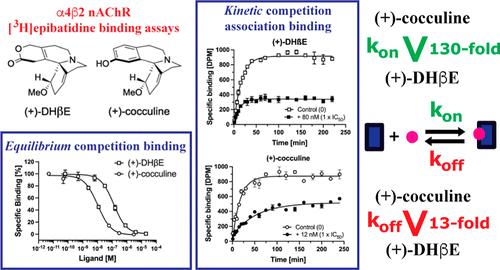
为了探索神经烟碱乙酰胆碱受体(nAChRs)的治疗潜力,已经开发了大量的激动剂和竞争性拮抗剂。基于平衡和动力学研究,我们报道了[3H]依巴替丁在5种异质αβ nachr上的动力学指纹图谱与7种经典激动剂在α4β2和α3β4 nachr上的动力学指纹图谱存在很大差异。虽然这种多样性取决于激动剂和受体亚型,但从这种分析中出现的动力学决定因素的总体模式是复杂的。两种生物碱和竞争拮抗剂(+)-DHβE和(+)-cocculine在α4β2 nAChR上的显著不同的结合动力学进一步说明了不同的动力学如何成为结构相似的类似物所表现出的非常相似的药理特性的基础。因此,我们的研究结果阐明了正位配体与αβ nachr结合的异质动力学基础,并强调了这些配体的结合亲和性、选择性和构效关系是如何根植于它们在受体上的动力学特性的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Heterogeneous Kinetic Origins of the Binding Properties of Orthosteric Ligands at Heteromeric Nicotinic Acetylcholine Receptors
A plethora of agonists and competitive antagonists have been developed to explore the therapeutic potential in neuronal nicotinic acetylcholine receptors (nAChRs). Based on equilibrium and kinetic [3H]epibatidine binding studies, we report that the kinetic fingerprints of [3H]epibatidine at five heteromeric αβ nAChRs and of seven classical agonists at α4β2 and α3β4 nAChRs differ substantially. While this diversity depends on both the agonist and receptor subtype, the overall pattern of kinetic determinants emerging from this profiling is complex. The dramatically different binding kinetics displayed by two alkaloids and competitive antagonists, (+)-DHβE and (+)-cocculine, at the α4β2 nAChR further exemplify how dissimilar kinetics can underlie very comparable pharmacological properties exhibited by close structural analogs. Thus, our findings elucidate the heterogeneous kinetic basis for orthosteric ligand binding to αβ nAChRs and emphasize how the binding affinities, selectivity profiles, and structure–activity relationships of these ligands are rooted in their kinetic traits at the receptors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


