森田-贝利斯-希尔曼醋酸酯与非活化脂肪酸的可见光介导的烯丙基脱羧烷基化反应。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
羧酸是一种稳定且易于获得的化学原料,是通过脱羧过程构建C(sp3)-C(sp3)键的最佳和基本合成平台。本文研究了一种可见光诱导、无金属的森田-贝利斯-希尔曼醋酸酯与脂肪酸直接脱羧烯丙基烷基化反应策略。该模型以优异的收率生产了一系列三取代烯烃。该方案具有广泛的底物范围,温和和氧化还原中性条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
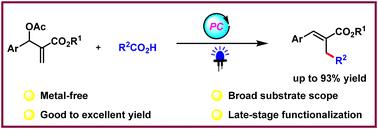
Visible light-mediated decarboxylative allylic alkylation of Morita–Baylis–Hillman acetates with unactivated aliphatic acids†
Carboxylic acids are bench-stable and readily available chemical feedstocks that function as optimal and fundamental synthetic platforms for the construction of C(sp3)–C(sp3) bonds through decarboxylation processes. Herein, a visible light-induced and metal-free strategy for the direct decarboxylative allylic alkylation of Morita–Baylis–Hillman acetates with aliphatic acids was developed. The model delivered a series of trisubstituted alkenes in good to excellent yields. This protocol features broad substrate scope, and mild and redox-neutral conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


