通过 NLRP6 跨领域检测肠道原生动物
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
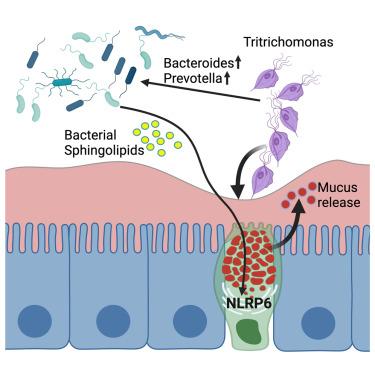
肠道原生生物通过宿主先天免疫系统检测,其机制尚不清楚。在这里,我们证明了原生毛滴虫以NLRP6-、ASC-和caspase-11依赖的方式诱导结肠粘液增厚,这与前哨杯状细胞的激活一致。黏液生长用感染毛滴虫的小鼠的盲肠提取物重现,而不是纯化的原生动物,这表明NLRP6可能检测到感染引起的微生物生态失调。与此一致的是,毛滴虫感染导致微生物群的变化,随着拟杆菌和普雷沃氏菌的扩张,非靶向代谢组学揭示了几种代谢物的急剧增加,包括鞘脂。最后,通过结合非生物小鼠和离体黏液分析,我们证明野生型但非鞘脂缺乏的B. thetaiotaomicron足以诱导nlrp6依赖性前哨杯状细胞功能,在雌性小鼠中观察到的效果最大。因此,我们提出NLRP6是通过监测微生物鞘脂来监测肠道原生动物感染的传感器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cross-kingdom-mediated detection of intestinal protozoa through NLRP6
Intestinal protists are detected by the host innate immune system through mechanisms that remain poorly understood. Here, we demonstrate that Tritrichomonas protozoa induce thickening of the colonic mucus in an NLRP6-, ASC-, and caspase-11-dependent manner, consistent with the activation of sentinel goblet cells. Mucus growth is recapitulated with cecal extracts from Tritrichomonas-infected mice but not purified protozoa, suggesting that NLRP6 may detect infection-induced microbial dysbiosis. In agreement, Tritrichomonas infection causes a shift in the microbiota with the expansion of Bacteroides and Prevotella, and untargeted metabolomics reveals a dramatic increase in several classes of metabolites, including sphingolipids. Finally, using a combination of gnotobiotic mice and ex vivo mucus analysis, we demonstrate that wild-type, but not sphingolipid-deficient, B. thetaiotaomicron is sufficient to induce NLRP6-dependent sentinel goblet cell function, with the greatest effect observed in female mice. Thus, we propose that NLRP6 is a sensor of intestinal protozoa infection through monitoring microbial sphingolipids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


