羰基催化的分子内葡萄链反应制备邻菲咯啉衍生物的设计与开发
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究揭示了n -杂环碳(NHC)催化的分子内葡萄系Stetter反应的设计和开发。该方法可以合成各种苯咯罗衍生物和萘酚-杂环化合物,收率非常高。该反应的成功依赖于双芳基醛的设计,对醌甲基(p-QM)部分作为葡萄状Stetter(1,6-共轭)受体,而醛官能团作为酰基阴离子等价物,由nhc催化的unpolung原位生成。该方案的突出特点包括原子效率、操作简单、底物范围广、大规模合成和合成后修饰。本文章由计算机程序翻译,如有差异,请以英文原文为准。
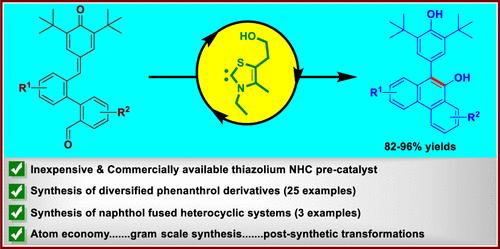
Design and Development of Carbene-Catalyzed Intramolecular Vinylogous Stetter Reaction to Access Phenanthrol Derivatives
The present study reveals the design and development of an N-heterocyclic carbene (NHC)-catalyzed intramolecular vinylogous Stetter reaction. This protocol enabled the synthesis of diverse phenanthrol derivatives and naphthol-fused heterocycles in very good to excellent yields. The success of the title reaction relies on the design of biaryl aldehydes bearing p-quinone methide (p-QM) moiety that acts as a vinylogous Stetter (1,6-conjugate) acceptor while the aldehyde functional group serves as an acyl anion equivalent, generated in situ from NHC-catalyzed umpolung. Salient features of the presented protocol include atom efficiency, operational simplicity, broad substrate scope, large-scale synthesis, and postsynthetic modifications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


