通过串联水氧化实现乙烯电化学转化的单原子 M/GDY 催化剂
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电催化为乙烯在常温条件下氧化生成高价值的含氧产物提供了一条很有前途的途径,有助于平衡化石能源消耗和环境保护之间的关系。然而,乙烯氧化过程需要与析氧反应(OER)相结合。在本研究中,采用第一性原理计算系统地研究了负载型单原子催化剂M/GDY在oer辅助下催化乙烯氧化的性能。我们的研究重点是利用金属位点的调整来影响反应中间体的吸附和活化,从而调节乙烯氧化的反应途径和最终产物的选择性。结果表明,Co、Cu和Rh金属位有效地稳定了*OHCH2-CH2OH中间体,促进了乙二醇氧化产物(HCOOH和OHCH2-COOH)的形成。值得注意的是,Rh/GDY催化剂在0.60 eV的低电压下表现出优异的催化性能。电子结构分析表明,乙烯π电子与金属原子之间的相互作用是乙烯活化的关键。该研究为研究乙烯氧化机理提供了新的思路,并为高效电催化剂的开发提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。

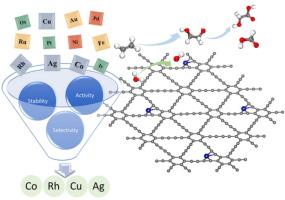
Single-atom M/GDY catalysts for electrochemical ethylene conversion via tandem water oxidation
Electrocatalysis offers a promising approach for the oxidation of ethylene to high-value oxygenated products under ambient conditions, contributing to the balance between fossil energy consumption and environmental protection. However, the ethylene oxidation process requires conjunction with the oxygen evolution reaction (OER). In this study, first-principles calculations are employed to systematically investigate the catalytic performance of supported single-atom catalysts M/GDY for ethylene oxidation in OER-assisted. Our study focuses on using the adjustment of metal sites to influence the adsorption and activation of reaction intermediates, thereby regulating the reaction pathways and selectivity of final products in ethylene oxidation. The results show that Co, Cu, and Rh metal sites effectively stabilize the *OHCH2-CH2OH intermediate, promoting the formation of ethylene glycol oxidation products (HCOOH and OHCH2-COOH). Notably, the Rh/GDY catalyst exhibits excellent catalytic performance with a low voltage of 0.60 eV. Electronic structure analysis reveals that the interaction between the π electrons of ethylene and the metal atoms is crucial for ethylene activation. This study provides new insights into the mechanisms of ethylene oxidation and offers guidance for the development of efficient electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


