环氧/氰酸酯共固化体系的固化动力学及时间-温度转变固化图研究
IF 4.5
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
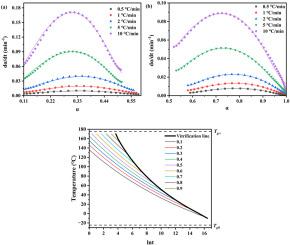
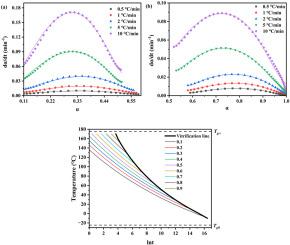
环氧树脂与氰酸酯的共聚结合了环氧热固性树脂和氰酸酯的优点,在电子封装和航空航天应用领域发挥着重要作用。然而,由于网状聚合物的反应机理复杂,且其生长呈阶梯状,因此最优的固化控制是实现优异性能的关键。虽然许多研究小组对环氧/氰酸酯共固化体系的固化行为进行了研究,但这些研究主要集中在应用不同的模型来获得不同的固化动力学参数。本文除了利用Kissinger方程和Ozawa方程获得固化动力学参数外,还特意在Kamal模型中引入扩散因子,成功地描述了整个固化过程,包括动力学控制阶段和扩散控制阶段。同时,在环氧/氰酸酯共固化体系反应机理复杂的情况下,证实了DiBenedetto方程对Tg与转化率曲线的适用性,并观察了Tg与共固化体系转化率之间的一对一关系。此外,研究了时间-温度-叠加动力学,得到了相应的Tg ~ lnt主曲线。首次构建了环氧/氰酸酯共固化体系的时间-温度转变固化图,包括玻璃化曲线和等转化曲线。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A study on cure kinetics and time-temperature-transformation cure diagram of epoxy/cyanate ester co-curing system
Copolymerization of epoxy resin and cyanate ester combines the advantages of both epoxy thermosets and cyanate esters, taking important roles in the field of electronic packaging and aerospace applications. However, optimal cure control is essential to achieve outstanding performance, due to its complex reaction mechanism and step growth of network polymer. Although many groups investigated the cure behaviors of epoxy/cyanate ester co-curing system, those works are mainly focused on applying different models to obtain various curing kinetic parameters. Herein, in addition to obtaining curing kinetic parameters using Kissinger equation and Ozawa equation, diffusion factor was purposely introduced into Kamal model to successfully describe the whole cure process, including both kinetically controlled stage and diffusion controlled stage. Meanwhile, the applicability of DiBenedetto equation to Tg vs. conversion plots of epoxy/cyanate ester co-curing system was confirmed regardless of its complex reaction mechanism, and the one-to-one relationship between Tg and conversion of this co-curing system was observed. In addition, time-temperature-superposition kinetic was studied, and corresponding Tg ∼ lnt master curve was obtained. Moreover, time-temperature-transformation cure diagram, with vitrification curve and iso-conversion curves, was constructed for the first time in the case of epoxy/cyanate ester co-curing system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


