IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
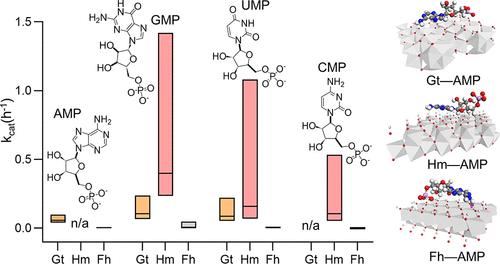
铁氧化物是有文献记载的磷(P)吸附剂,已被证明可催化有机磷的脱磷作用,从而使这些矿物成为磷循环中的催化陷阱。然而,目前还缺乏对这种非生物催化作用的定量评估。在这里,我们研究了八种核糖核苷酸与普通氧化铁反应的去磷酸化动力学,这些核糖核苷酸具有不同的核碱基结构和钾当量。X 射线吸收光谱确定,0-98% 的矿物质结合 P 是回收的无机 P (Pi)。基质辅助激光解吸/电离质谱法证明了与鹅铁矿结合的短寿命三磷酸化和单磷酸化核糖核苷酸。根据对溶解的π和矿物结合的π的动力学演化进行的迈克尔斯-门顿(Michaelis-Menten)型建模,三磷酸化核糖核苷酸与鹅辉石反应产生π的最大速率(1.9-16.1 μmol Pi h-1 ggoethite-1)比与赤铁矿和铁酸盐反应产生π的速率高5倍;单磷酸化核糖核苷酸只产生矿物结合的π,不同矿物产生π的速率相似(0.0-12.9 μmol Pi h-1 gmineral-1)。嘌呤核糖核苷酸和嘧啶核糖核苷酸之间没有明显区别。根据矿物的 Pi 结合能力进行归一化处理后,得出的催化周转率意味着表面化学控制的反应性。通过红外光谱和分子建模确定了核糖核苷酸与矿物的复合机制。我们估计土壤中氧化铁催化的速率(0.01-5.5 μmol Pi h-1 gsoil)与已报道的土壤磷酸酶速率相当,这表明矿物和酶都是钾循环中的相关催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quantitative Benchmarking of Catalytic Parameters for Enzyme-Mimetic Ribonucleotide Dephosphorylation by Iron Oxide Minerals
Iron oxides, which are documented phosphorus (P) sinks as adsorbents, have been shown to catalyze organic P dephosphorylation, implicating these minerals as catalytic traps in P cycling. However, quantitative evaluation of this abiotic catalysis is lacking. Here, we investigated the dephosphorylation kinetics of eight ribonucleotides, with different nucleobase structures and P stoichiometry, reacting with common iron oxides. X-ray absorption spectroscopy determined that 0–98% of mineral-bound P was recycled inorganic P (Pi). Matrix-assisted laser desorption/ionization with mass spectrometry demonstrated short-lived triphosphorylated and monophosphorylated ribonucleotides bound to goethite. Based on Michaelis-Menten type modeling of the kinetic evolution of both dissolved and mineral-bound Pi, maximal Pi production rates from triphosphorylated ribonucleotides reacted with goethite (1.9–16.1 μmol Pi h–1 ggoethite–1) were >5-fold higher than with hematite and ferrihydrite; monophosphorylated ribonucleotides generated only mineral-bound Pi at similar rates (0.0–12.9 μmol Pi h–1 gmineral–1) across minerals. No clear distinction was observed between purine-based and pyrimidine-based ribonucleotides. After normalization to mineral-dependent Pi binding capacity, resulting catalytic turnover rates implied surface chemistry-controlled reactivity. Ribonucleotide–mineral complexation mechanisms were identified with infrared spectroscopy and molecular modeling. We estimated iron oxide-catalyzed rates in soil (0.01–5.5 μmol Pi h–1 gsoil) comparable to reported soil phosphatase rates, highlighting both minerals and enzymes as relevant catalysts in P cycling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


